- Vinblastine
-

- $110.00 / 1kg
-
2023-02-13
- CAS:865-21-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100MT
- Vinblastine
-

- $15.00 / 1KG
-
2021-07-13
- CAS:865-21-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Vinblastine
-

- $15.00 / 1KG
-
2021-07-09
- CAS:865-21-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | VINBLASTINE Basic information |
| Product Name: | VINBLASTINE | | Synonyms: | VINBLASTINE 94% BP98;VINBLASTINE;VINBLASTINE SULFATE(RG);[3aR-[3aa,4b,5b,5ab,9(3R*,5S*,7R*,9S*),10bR*,13aa]]-Methyl 4-(acetyloxy)-3a-ethyl-9-[5-ethyl-1,4,5,6,7,8,9,10-octahydro-5-hydroxy-9-(methoxycarbonyl)-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-3a,4,5,5a,6,11,12,13a-octahydro-5-hydroxy-8-methoxy-6-methyl-1H-indolizino[8,1-cd]carbazole-5-carboxylate;1H-Indolizino[8,1-cd]carbazole, vincaleukoblastine deriv.;1H-Indolizino[8,1-cd]carbazole-5-carboxylic acid, 4-(acetyloxy)-3a-ethyl-9-[5-ethyl-1,4,5,6,7,8,9,10-octahydro-5-hydroxy-9-(methoxycarbonyl)-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-3a,4,5,5a,6,11,12,13a-octahydro-5-hydroxy-8-methoxy-6-methyl-, methyl ester, [3aR-[3aa,4b,5b,5ab,9(3R*,5S*,7R*,9S*),10bR*,13aa]]-;2H-3,7-Methanoazacycloundecino[5,4-b]indole, vincaleukoblastine deriv.;Rozevin | | CAS: | 865-21-4 | | MF: | C46H58N4O9 | | MW: | 810.99 | | EINECS: | 212-734-0 | | Product Categories: | phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Inhibitors;chemical reagent;pharmaceutical intermediate | | Mol File: | 865-21-4.mol | 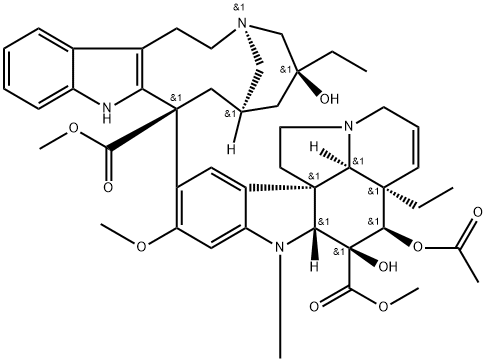 |
| | VINBLASTINE Chemical Properties |
| Melting point | 211-216° | | alpha | D23 -32° (c = 0.88 in methanol) | | Boiling point | 755.65°C (rough estimate) | | density | 1.1325 (rough estimate) | | refractive index | 1.6000 (estimate) | | storage temp. | Sealed in dry,Store in freezer, under -20°C | | solubility | Chloroform, Dichloromethane, Dimethyl Sulfoxide, Methanol | | form | Solid | | pka | 5.4, 7.4(at 25℃) | | color | Yellow | | EPA Substance Registry System | Vinblastine (865-21-4) |
| | VINBLASTINE Usage And Synthesis |
| Description | An alkaloid derived
from the periwinkle, Catharanthus roseus (formerly Vinca
rosea) used as an antitumor drug in the treatment of Hodgkins
lymphoma and other cancers. It binds to the microtubule subunit, tubulin, at a site distinct from that of colchicine and
podophyllotoxin. This binding is reversible, temperature
dependent and rapid, and results in large tubulin aggregates of
highly ordered structure (vinblastine paracrystals). | | Physical properties | Appearance: needlelike crystals were produced when recrystallized by methanol.
Solubility: soluble in chloroform, acetone, and ethanol. Melting point: 211–
216?°C.?Sulfate melting point of vinblastine is 284–285?°C; hydrochloric acid melting point of vinblastine is 244–246?°C (decomposes). Specific optical rotation:
+42°. | | Originator | Velban ,Lilly ,US ,1961 | | Uses | Vinblastine is used for severe lymphoblastic leukemia, Hodgkin’s disease, non�Hodgkin’s lymphoma, neuroblastoma, sarcoma, and other cancerous diseases. | | Uses | Labelled Vinblastine (V314000). Antitumor alkaloid isolated from periwinkle, Vinca rosea Linn., Apocynaceae; inhibits microtubule assembly. An antineoplastic. | | Indications | It was recorded in the Pharmacopoeia of the People’s Republic of China (2015), the
British Pharmacopoeia (2017), the United States Pharmacopeia (40), the Japanese
Pharmacopoeia (17th ed.), the European Pharmacopoeia (9th ed.), and The
International Pharmacopoeia (5th ed.).
Vinblastine sulfate injection is used as the first-line therapy to treat Hodgkin
lymphoma, lymphocytic leukemia, testis tumor, and choriocarcinoma intravenously
in clinical practice generally according to ABVD methods (Adriamycin, bleomycin,
vinblastine, and dacarbazine). | | Manufacturing Process | According to US Patent 3,225,030, 1,500 grams of dried ground plant of Vinca
rosea were intimately mixed with 1,000 ml of a 2% tartaric acid solution, and
the mixture was extracted with three 9-liter portions of benzene. The benzene
extracts were combined and were concentrated in vacuo to about 1,500 ml.
The concentrate was mixed with 1 liter of 2% tartaric acid and the mixture
was steam-distilled under reduced pressure until all of the benzene had
distilled over. The insoluble residue was dissolved in hot methanol, a second
1-liter portion of 2% tartaric acid solution was added, and the mixture was
steam-distilled under reduced pressure until all of the methanol had distilled.
The undistilled aqueous tartaric acid solution was extracted with three 1-liter
portions of ethylene dichloride, and was then brought to a pH of about 8.5 to
9.5 by the addition of 28% aqueous ammonium hydroxide. The ammoniacal
solution was extracted with three 1-liter portions of ethylene dichloride; the
ethylene dichloride extracts were combined, were dried, and were evaporated
in vacuo, yielding a residue of 3.35 grams of a light-brown powder.
1 1/2 grams of the residue were dissolved in 10 ml of benzene, and the
solution was passed over a chromatographic adsorption column containing 50
grams of alumina (Alcoa activated alumina, Grade F-20) which had previously
been shaken for about 20 minutes with a mixture of 100 ml of benzene
containing 1.5 ml of 10% acetic acid.
The column was developed by washing it with 2,100 ml of benzene. The
column was then washed sequentially with 300 ml of benzene-chloroform
solvent (95:5 by volume) and 800 milliliters of benzene-chloroform solvent
(75:25) to remove indeterminate impurities. The leurosine was eluted from
the alumina by passing over the column 900 ml of benzene chloroform solvent
(50:50).
The eluate was evaporated to dryness in vacuo, leaving an amorphous residue
of 113 mg of leurosine. The residue was treated with a few ml of methanol in
which it quickly dissolved, but from which leurosine quickly precipitated in
crystalline form. Because of the affinity of leurosine for water, and the
presence of traces of water in the solvents, the leurosine was obtained in the
form of its octahydrate. Although the material as obtained was substantially pure, it was further purified by recrystallizing it from hot methanol solution.
The hydrated leurosine obtained decomposed at about 200° to 205°C.
Further elution of the above chromatographic column with a 50:50 benzene-
chloroform solvent mixture or with a 25:75 benzene-chloroform solvent
mixture serves to elute vincaleukoblastine. Vincaleukoblastine also occurs in
the latter fractions containing leurosine. Vincaleukoblastine is obtained from
vincaleukoblastine-containing fractions by evaporation to dryness, either of a
filtrate from which leurosine has previously been isolated, or from a
chromatographic eluate fraction. The resulting residue is dissolved in ethanol
and 286 ethanolic sulfuric acid is added until the pH is lowered to about 4.
The solution is seeded with crystals of vincaleukoblastine sulfate and is chilled
for about 24 hours. Vincaleukoblastine sulfate, if present, precipitates during
this period and can be separated by filtration. Vincaleukoblastine sulfate melts
at about 284° to 285°C. | | Brand name | Velban (Lilly). | | Therapeutic Function | Cancer chemotherapy | | Mechanism of action | Vinblastine suppresses cell growth during metaphase, affects amino acid metabolism, in
particular at the level of including glutamine acid into the citric acid cycle and preventing
it from transformation into urea, and it also inhibits protein and nucleic acid synthesis. | | Pharmacokinetics | Leukopenia is the dose-limiting side
effect, and dose reductions are warranted in patients with serum bilirubin levels greater than 3 mg/dL. The
drug-related impact on erythrocyte and thrombocyte levels usually is insignificant. Like vincristine, it is
administered as an IV bolus or infusion. The initial elimination half-life of 3.7 minutes is similar to vincristine,
but the 24.8-hour terminal half-life is significantly shorter. | | Clinical Use | In addition to the hematologic indications that it shares with vincristine, vinblastine sulfate has
found utility in the treatment of advanced testicular carcinoma (often in combination with bleomycin),
advanced mycosis fungoides, Kaposi's sarcoma, and histiocytosis X. |
| | VINBLASTINE Preparation Products And Raw materials |
|