- Niraparib
-
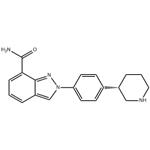
- $8.00 / 1kg
-
2024-04-08
- CAS:1038915-60-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Niraparib
-

- $0.00/ kg
-
2023-12-26
- CAS:1038915-60-4
- Min. Order: 1kg
- Purity: 99%, Single impurity<0.1
- Supply Ability: 1 ton
- Niraparib
-
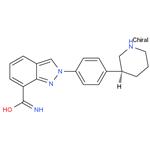
- $0.00 / 1kg
-
2023-11-01
- CAS:1038915-60-4
- Min. Order: 1kg
- Purity: 99.0%
- Supply Ability: 20 tons
|
| | Niraparib Basic information | | Uses |
| Product Name: | Niraparib | | Synonyms: | MK-4827,(S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-carboxaMide;Niraparib;2-[4-((3S)-3-Piperidinyl)phenyl]-2H-indazole-7-carboxamide;MK4827 (Niraparib);2H-Indazole-7-carboxamide, 2-[4-(3S)-3-piperidinylphenyl]-;(S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-carboxamide hydrocholoride;2-[4-(3S)piperidin-3-ylphenyl]-2H-indazol-7-carboxamide;2-[4-[(3s)-piperidin-3-yl]phenyl]indazole-7-carboxamide | | CAS: | 1038915-60-4 | | MF: | C19H20N4O | | MW: | 320.39 | | EINECS: | | | Product Categories: | Anti-cancer&immunity;API | | Mol File: | 1038915-60-4.mol | 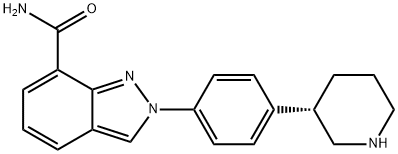 |
| | Niraparib Chemical Properties |
| Melting point | 187-189°C | | Boiling point | 463.6±45.0 °C(Predicted) | | density | 1.34 | | storage temp. | 2-8°C(protect from light) | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | 15.36±0.30(Predicted) | | form | Solid | | color | Pale Yellow to Light Yellow |
| | Niraparib Usage And Synthesis |
| Uses | Niraparib is a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. | | Description | (S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-carboxamide is also known as MK-4827(Niraparib) tosylate is a selective inhibitor of PARP1/PARP2 (The poly(ADP-ribose) polymerase) with great activity in cancer cells with mutant BRCA-1 and BRCA-2. It has been recently approved by FDA for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy. In vitro studies have shown that niraparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, apoptosis and cell death. | | Uses | Niraparib is a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. | | Definition | ChEBI: Niraparib is a 2-[4-(piperidin-3-yl)phenyl]-2H-indazole-7-carboxamide that has S-configuration. It is a potent inhibitor of PARP1 and PARP2 (IC50 of 3.8 and 2.1 nM, respectively) and approved as a first-line maintenance treatment for women with advanced ovarian cancer after responding to platinum-based chemotherapy. It has a role as an antineoplastic agent, an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor, a radiosensitizing agent and an apoptosis inducer. | | Pharmacology | The synthesis and initial pharmacology of niraparib have been published. Niraparib has affinity for PARP 1 and 2 inhibition (IC50 = 3.8 and 2.1 nM, respectively) and inhibits the proliferation of cancer cells with mutant BRCA1 and BRCA2 with IC50 values in the 10–100 nM range in vitro. Niraparib demonstrated efficacy as a single agent in a xenograft model of BRCA1-deficient cancer. Niraparib has also been reported to act as a preclinical radiosensitiser and has entered into clinical oncology trials. | | in vitro | mk-4827displayed excellent parp 1 and 2 inhibition with ic50 of 3.8 and 2.1 nm, respectively. in a whole cell assay, mk-4827 inhibited parp activity with ec50 of 4 nm. mk-4827 also inhibited proliferation of cancer cells with mutant brca-1 and brca-2 with cc50 in the 10-100 nm range[1]. | | in vivo | in a variety of human tumor xenografts of differing p53 status,mk-4827 showed high potential to improve the efficacy of radiotherapy,such as calu-6 (p53 null), a549 (p53 wild-type [wt]) and h-460 (p53 wt) lung cancers and triple negative mda-mb-231 human breast carcinoma [3]. | | References | https://newdrugapprovals.org/2016/12/22/niraparib-mk-4827/
https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm548487.htm
https://www.drugbank.ca/drugs/DB11793
Sandhu, Shahneen K, et al. "The poly (ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA, mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial." Lancet Oncology14.9 (2013):882.
Jones, P, et al. "Niraparib: A Poly (ADP-ribose) Polymerase (PARP) Inhibitor for the Treatment of Tumors with Defective Homologous Recombination. " Journal of Medicinal Chemistry 58.8(2015):3302-14. |
| | Niraparib Preparation Products And Raw materials |
|