- Furaltadone
-

- $0.00 / 25KG
-
2023-08-31
- CAS:139-91-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Furaltadone
-

- $200.00 / 1KG
-
2021-10-20
- CAS:139-91-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 9000kg/per week
- Furaltadone
-

- $10.00 / 1KG
-
2021-09-02
- CAS:139-91-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
|
| | Furaltadone Basic information |
| Product Name: | Furaltadone | | Synonyms: | FURALTADONE VETRANAL;FURALTADONE FREE BASE;5-MORPHOLINOMETHYL-3-(5-NITROFURFURYLIDENEAMINO)OXAZOLIDONE;Furaltadone (base and/or unspecified salts);5-(4-Morpholinylmethyl)-3-[[(5-nitro-2-furanyl)methylene]amino]-2-oxazolidinone;5-(Morpholinomethyl)-3-(5-nitrofurfurylidenamino)-2-oxazolidone;altafur;f-150 | | CAS: | 139-91-3 | | MF: | C13H16N4O6 | | MW: | 324.29 | | EINECS: | 205-384-5 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 139-91-3.mol | 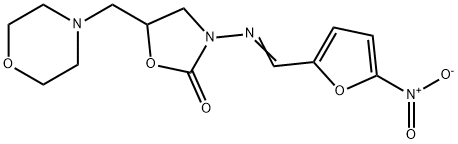 |
| | Furaltadone Chemical Properties |
| Melting point | 206°C (dec.) | | Boiling point | 462.63°C (rough estimate) | | density | 1.3046 (rough estimate) | | refractive index | 1.6120 (estimate) | | storage temp. | Sealed in dry,2-8°C | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | pka | pKa 5.0 (Uncertain) | | color | Yellow | | BRN | 8130725 | | Stability: | Light sensitive | | EPA Substance Registry System | Furaltadone (139-91-3) |
| Hazard Codes | Xn,Xi | | Risk Statements | 22-36/37/38 | | Safety Statements | 3-26 | | RIDADR | 3249 | | WGK Germany | 3 | | RTECS | RQ3620000 | | HazardClass | 6.1(b) | | PackingGroup | III | | Toxicity | mouse,LD50,intraperitoneal,1gm/kg (1000mg/kg),LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSIONKIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITIONBEHAVIORAL: ANTIPSYCHOTIC,Journal of Pharmacy and Pharmacology. Vol. 16, Pg. 663, 1964. |
| | Furaltadone Usage And Synthesis |
| Description | This nitrofuran derivative can be added to animal feed.
It induced contact dermatitis in a farm employee. | | Chemical Properties | Yellow Crystalline Solid | | Originator | Altafur,Norwich Eaton,US,1959 | | Uses | Furaltadone is a metabolite of Nitrofuran in milk and an intermediate in the synthesis of AMOZ (A634600), a metabolite of Furaltadone Hydrochloride. | | Uses | A metabolite of Nitrofuran in milk. Antibacterial | | Definition | ChEBI: An oxazolidinone that is 1,3-oxazolidin-2-one substituted at position 1 by a (5-nitro-2-furyl)methylene]amino group and at position 5 by a morpholin-4-ylmethyl group. An antibacterial formerly used oraly but withdrawn due to toxicity, it is used topically
as the hydrochloride salt) for treatment of ear disorders. | | Manufacturing Process | 11.17 g (0.78 mol) 3-(N-morpholinyl)-1,2-epoxypropane, BP 76.5°C to 78°C.
3.9 mm, prepared by Eisleb's method for 3-(1-piperidyl)-1,2-epoxypropane
(US Patent 1,790,042) is added dropwise in 12 minutes to 19.5 g (0.39 mol)
100% hydrazine hydrate, which has been warmed to 85% on the steam bath,
and is being mechanically stirred. The heat of the reaction maintains the
internal temperature at 90°C to 100°C without further external heating. The
reaction mixture is then warmed on the steam bath for an additional two
hours (90°C to 95°C). The excess hydrazine hydrate is removed in vacuo. The
residue of viscous 1-hydrazino-3-morpholinyl-2-propanol is not distilled, but is
mixed with 10.16 g (0.086 mol) diethyl carbonate and a solution of 0.3 g
sodium metal in 15 ml methyl alcohol. The mixture is refluxed about 2 hours
under a 15 cm Widmer column, the alcohol being removed leaving a thick,
green liquid residue, which is cooled and the precipitate which forms is
removed by filtration and washed well with ether. Yield 82%. MP 114°C to
116°C. Recrystallization from isopropanol gives purified 3-amino-5-(N�morpholinyl)-methyl-2-oxazolidone, MP 120°C as the intermediate.
It is not necessary that the intermediate be separated from the reaction
medium in the preparation of the end product. Instead, the reaction mixture,
after cooling, is treated with 200 ml of water acidified with 42 ml 10% hydrochloric acid solution, and filtered. To the clear, light yellow filtrate is
added dropwise a solution of 9.8 g (0.07 mol) 5-nitro-2-furaldehyde in 100 ml
ethyl alcohol. An orange solution of the hydrochloride results. The free base is
precipitated as yellow plates by making the solution basic with saturated
sodium carbonate solution. 14 g of the compound is filtered off by suction,
washed with alcohol, and dried. The yield, MP 204°C to 205°C (dec.), is 53%
of theoretical based on 3-(N-morpholinyl)-1,2-epoxy-propane.
Recrystallization from 95% alcohol (75%recovery) raises the melting point to
206°C (dec.).
The hydrochloride salt is isolated quantitatively by suspending the base in
alcohol and adding sufficient aqueous concentrated HCl solution. The
precipitate becomes pale yellow, is filtered off, and recrystallized from 80%
alcohol. The MP range is about 223°C to 228°C (dec.). | | Therapeutic Function | Antibacterial | | Contact allergens | This nitrofuran derivative can be added in animal feed
or in eardrops |
| | Furaltadone Preparation Products And Raw materials |
|