- Thienamycin USP/EP/BP
-
- $1.10 / 1g
-
2021-06-22
- CAS:59995-64-1
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
- Thienamycin
-

- $1.00 / 1g
-
2019-12-26
- CAS:59995-64-1
- Min. Order: 1g
- Purity: ≥98%
- Supply Ability: g/kg/Ton
|
| | Thienamycin Basic information |
| Product Name: | Thienamycin | | Synonyms: | [SR-[5α,6α(R^<*>^)]]-3-[(2-Aminoethyl)thio]-6-(1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;(5R)-3-(2-Aminoethylthio)-6β-[(R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hepta-2-ene-2-carboxylic acid;(5R)-3-[(2-Aminoethyl)thio]-6β-[(R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;(5R,6R)-3-(2-aminoethylsulfanyl)-6-[(1R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;(5R,6R)-3-(2-aminoethylthio)-6-[(1R)-1-hydroxyethyl]-7-keto-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;(5R,6R)-3-(2-azanylethylsulfanyl)-6-[(1R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;IMipeneM IMpurity A;Imipenem Impurity 1(Imipenem EP Impurity A) | | CAS: | 59995-64-1 | | MF: | C11H16N2O4S | | MW: | 272.32 | | EINECS: | 203-170-6 | | Product Categories: | | | Mol File: | 59995-64-1.mol | 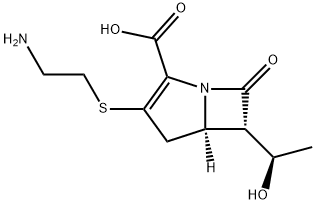 |
| | Thienamycin Chemical Properties |
| alpha | D27 +82.7° (c = 1.0 in water) | | Boiling point | 514.0±50.0 °C(Predicted) | | density | 1.50±0.1 g/cm3(Predicted) | | storage temp. | Hygroscopic, -86°C Freezer, Under inert atmosphere | | solubility | Chloroform (Slightly), Methanol (Slightly), Water (Slightly) | | form | Solid | | pka | 4.20±0.40(Predicted) | | color | Off-White to Dark Yellow | | Stability: | Hygroscopic, Temperature Sensitive, Very Unstable | | InChI | InChI=1S/C11H16N2O4S/c1-5(14)8-6-4-7(18-3-2-12)9(11(16)17)13(6)10(8)15/h5-6,8,14H,2-4,12H2,1H3,(H,16,17)/t5-,6-,8-/m1/s1 | | InChIKey | WKDDRNSBRWANNC-ATRFCDNQSA-N | | SMILES | N12[C@@]([H])([C@@H]([C@H](O)C)C1=O)CC(SCCN)=C2C(O)=O |
| | Thienamycin Usage And Synthesis |
| Description | Thienamycin, one of the most potent naturally-produced antibiotics known thus far, was discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. | | Uses | Thienamycin is one of the most potent naturally produced antibiotics known thus far. It has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. | | Definition | ChEBI: Thienamycin is a member of carbapenems. | | Biosynthesis | The formation of thienamycin is thought to occur through a different pathway from classic β-lactams (penicillins, cephalosporins). Production of classic β-lactams in both fungi and bacteria occur through two steps: First, the condensation of L-cysteine, L-Valine, and L-α-amino adipic acid by ACV synthetase (ACVS, a nonribsomal peptide synthetase) and then cyclization of this formed tripeptide by isopenicillin N synthetase (IPNS). | | Mechanism of action | In vitro, thienamycin employs a similar mode of action as penicillins through disrupting the cell wall synthesis (peptidoglycan biosynthesis) of various Gram-positive and Gram-negative bacteria (Staphylococcus aureus,Staphylococcus epidermidis, Pseudomonas aeruginosa to name a few). In a study carried out by Spratt et al., they found that, although thienamycin binds to all of the penicillin-binding proteins (PBP) in Escherichia coli, it preferentially binds to PBP-1 and PBP-2, which are both associated with the elongation of the cell wall. Unlike pencillins, which are rendered ineffective through rapid hydrolysis by the β-lactamase enzyme present in some strains of bacteria, thienamycin remains antimicrobially active. Neu et al. found that thienamycin displayed high activity against bacteria that were resistant to other β-lactamase stable compounds (cephalosporins), highlighting the superiority of thienamycin as an antibiotic among β-lactams. | | Clinical Use | Thienamycin itself is extremely unstable and decomposes in aqueous solution. Consequently, thienamycin is impractical for clinical treatment of bacterial infections. For this reason, stable derivatives of thienamycin were created for medicinal consumption. One such derivative, imipenem, was formulated in 1985. Imipenem, an N-formimidoyl derivative of thienamycin, is rapidly metabolized by the renal dihydropeptidase enzyme found in the human body. To prevent its rapid degradation, imipenem is normally co-administered with cilastatin, an inhibitor of this enzyme. |
| | Thienamycin Preparation Products And Raw materials |
|