|
ChemicalBook Optimization Suppliers |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
|
| 化学名: | L-メチオニン | | 英語化学名: | L-Methionine | | 别名: | L-Methionine, Animal-Free;L-METHIONINE (13C5,D8,15N);L-METHIONINE (1,2,3,4-13C4;METHYL-13CH3);L-METHIONINE, REAGENT GRADE, >=98% (;L-MethionineUSP,
98.5-101.5% (Titration: anhydrous basis);L(-)-Methionin;L-2-Amino-3-methylthiobuttersαure | | CAS番号: | 63-68-3 | | 分子式: | C5H11NO2S | | 分子量: | 149.21 | | EINECS: | 200-562-9 | | カテゴリ情報: | amino;Miscellaneous Compounds;Amino Acids & Derivatives;Sulfur & Selenium Compounds;fertilizer;Amino Acids;Chiral Compound;Methionine [Met, M];Amino Acids and Derivatives;Amino Acids;Amino Acid Derivatives;bc0001;63-68-3 | | Mol File: | 63-68-3.mol | 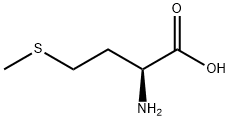 |
| 融点 | 284 °C (dec.)(lit.) | | 比旋光度 | 23.25 º (c=2, 6N HCl) | | 沸点 | 393.91°C (estimate) | | 比重(密度) | 1,34g/cm | | 屈折率 | 1.5216 (estimate) | | 貯蔵温度 | 20-25°C | | 溶解性 | 1 M HCl: 0.5 M at 20 °C, clear, colorless | | 外見 | Solid | | 酸解離定数(Pka) | 2.13(at 25℃) | | 色 | White | | PH | 5-7 (10g/l, H2O, 20℃) | | 臭い (Odor) | Slight characteristic | | 光学活性 (optical activity) | [α]20/D +23.7±0.5°, c = 5% in 5 M HCl | | 水溶解度 | Soluble | | 極大吸収波長 (λmax) | λ: 260 nm Amax: 0.40
λ: 280 nm Amax: 0.05 | | Merck | 14,5975 | | BRN | 1722294 | | 安定性: | Stable. Incompatible with strong oxidizing agents. | | LogP | 0.217 (est) | | CAS データベース | 63-68-3(CAS DataBase Reference) | | NISTの化学物質情報 | L-Methionine(63-68-3) | | EPAの化学物質情報 | L-Methionine (63-68-3) |
| | L-メチオニン Usage And Synthesis |
| 外観 | 白色, 結晶~結晶性粉末 | | 定義 | 本品は、次の化学式で表されるアミノ酸である。 | | 溶解性 | 水100gに3g (0℃), 5.6g (30℃)溶解。有機溶媒に不溶。酸, アルカリ溶液に可溶。水にやや溶けやすく、エタノールに極めて溶けにくく、ジエチルエーテルにほとんど溶けない。塩酸又は水酸化ナトリウム溶液に溶ける。 | | 解説 | L-メチオニン,(S)-2-amino-4-(methylthio)butanoic acid.C5H11NO2S(149.21).CH3SCH2CH2CH(NH2)COOH.略称MetまたはM.必須アミノ酸の一つで,量的には少ないが分布は広い.発酵法で得られるホモセリンから工業的に合成できる.また,D,L-体はβ-メチルチオアセトアルデヒドからストレッカー法で合成される.生体中でメチル基の供与体として重要であり,ほかのアミノ酸の前駆物質である.六角板状結晶.分解点280~281 ℃.[α]25D-8.2°(水).pK1 2.15,pK2 9.06(25 ℃).水に可溶,エタノール,エーテルに不溶.特異な臭気とわずかに甘みと苦みを有する.栄養剤,医薬として用いる.LD50 29 mmol/kg(ラット,腹腔内) | | 化粧品の成分用途 | ヘアコンディショニング剤、皮膚コンディショニング剤 | | 効能 | 解毒薬 | | 説明 | Colorless or white lustrous plates, or a white crystalline powder.
It has a slight, characteristic odor. It is soluble in water, in alkali
solutions, and in dilute mineral acids. It is slightly soluble in
alcohol and practically insoluble in ether. | | 化学的特性 | Methionine occurs as a white or almost white, crystalline powder or
colorless crystals. | | 化学的特性 | White crystalline powder | | Originator | Meonine ,Ives,US,1944 | | 使用 | Essential aminoacid for human development. Hepatoprotectant; antidote (acetominophen poisoning); urinary acidifier. | | 使用 | methionine is slows down and normalizes oil gland sebum production. Methionine is also used as a texturizer in cosmetic creams. It is an essential amino acid found in a number of proteins and obtained by means of fermentation. | | 使用 | L-Methionine is used as an essential amino acid for human development and therapeutically acts as an antidote for acetaminophen poisoning. It serves as a chelating agent for heavy metals, as a flavoring agent and nutritional supplement for foods. It acts as a feed additive, vegetable oil enrichment and as a single cell protein. In addition to this, it is used as a hepatoprotectant and also acts as a lipotropic agent and prevents excess fat buildup in the liver. | | 定義 | ChEBI: The L-enantiomer of methionine. | | 製造方法 | The production method of choice for L-methionine is still the enzymatic resolution of racemic N-acetyl-methionine using acylase from Aspergillus oryzae. The production is carried out in a continuously operated fixed-bed or enzyme membrane reactor. | | 調製方法 | Numerous methods have been described for manufacture of
methionine, including hydrolysis of methionine amide )and 5-(bmethylmercaptoethyl)-
hydantoin. | | Manufacturing Process | A 3-necked flask fitted with a stirrer, thermometer, gas inlet, dropping funnel, and brine-cooled reflux condenser was charged with 53 g (1.1 mol) methyl mercaptan and 0.35 g mercuric methyl mercaptide. After admitting 56 g (1.0mol) of acrolein during the course of 15 minutes with an inside temperature of
about 10°C, the temperature was allowed to rise spontaneously to 75°C, at
which point an ice bath was applied. There was no indication of further
reaction one hour after the addition of the acrolein. Distillation of the product
gave 71 g (yield 68%) of β-methylmercaptopropionaldehyde, as described in
US Patent 2,584,496.
Then as described in US Patent 2,732,400, β-methylmercaptopropionaldehyde
(0.60 M) (56.5 g) is added to a stirred solution of sodium cyanide (0.66 M)
(32.4 g) and ammonium chloride (0.63 M) (33.7 g) in water (140 ml). The
temperature of the mixture rises to 49°C and is maintained at this point by
heat evolution for about 5 minutes when it slowly begins to fall. Methanol (50
ml) is added and the mixture is stirred for 4 hours as the temperature falls to
28°C (room temperature).
After chilling to +12°C, additional methanol (35 ml) and a concentrated
aqueous ammoniun hydroxide solution (1.4 M) (100 ml) are added and
stirring is continued for 2 hours at a temperature maintained at from +5° to
+15°C. The organic layer is separated and solvent is stripped from the
aqueous layer at water aspirator pressure at a temperature below 40°C. The
residue is extracted several times with chloroform and the chloroform extracts
are combined with the separated oil. Chloroform is removed at water aspirator
pressure at a temperature below 35°C to leave crude α-amino-γmethylmercaptobutyronitrile (methionine nitrile) in 88% yield (68 g) as a
clear, somewhat viscous oil.
The methionine nitrile (20 g) is dissolved in a solution prepared from 50 ml of
aqueous 5 N sodium hydroxide solution and 65 ml of ethanol. The solution is
then refluxed for 24 hours; ammonia is evolved. The solution is treated with
activated carbon, filtered, acidified with glacial acetic acid (17 ml), chilled to -
10°C and filtered to give crude product. This crude product is then slurried
with a solution made up of 20 ml of water and 20 ml of methanol, filtered at -
5° to +10°C and dried to give dl-methionine as white platelets. | | Therapeutic Function | Lipotropic | | Synthesis Reference(s) | Canadian Journal of Chemistry, 47, p. 3271, 1969 DOI: 10.1139/v69-542
Synthetic Communications, 26, p. 3619, 1996 DOI: 10.1080/00397919608003774 | | 一般的な説明 | Minute hexagonal plates from dilute alcohol. | | 空気と水の反応 | Reacts with water, steam, and/or acids to produce toxic and flammable vapors of hydrogen sulfide . Water soluble . pH of 1% aqueous solution is 5.6-6.0. | | 反応プロフィール | An organosulfide and amine derivative, carboxylic acid. Look at Reactive Groups 20 (organosulfides), 7 (amines), and 3 (carboxylic acids) may give indications about reactive tendencies. L-Methionine is an amino acid essential in human nutrition. | | 健康ハザード | ACUTE/CHRONIC HAZARDS: L-Methionine is dangerous when heated to decomposition; it emits dangerous and highly toxic fumes. | | 火災危険 | Flash point data for L-Methionine is not available, but L-Methionine is probably combustible. | | 応用例(製薬) | Methionine is used in oral pharmaceutical formulations as a
flavoring agent.It has been included in parenteral formulations as
a pH controlling agent,and it has also been used experimentally
as an antioxidant with antibodies.Methionine is also used
therapeutically in oral tablets | | Biochem/physiol Actions | L-Methionine serves as precursor for transmethylation and transsulphuration. Methionine adenosylation results in the formation of S-adenosyl-L-methionine (SAM). SAM serves as a methyl donor to a number of substances. This methylation is significantly associated with the immune system functioning. Thus, SAM deficiency causes severe combined immunodeficiencies. L-Methionine′s metabolic product, glutathione, is known to regulate immune response and has antiviral action. | | 安全性プロファイル | Mildly toxic by
ingestion and intraperitoneal routes. Human
mutation data reported. An experimental
teratogen. Experimental reproductive
effects. An essential sulfur-containing amino
acid. When heated to decomposition it emits
very toxic fumes of NOx and SOx. | | 安全性 | Methionine is used in oral pharmaceutical formulations. The pure
form of methionine is mildly toxic by ingestion and by the IP route.
LD50 (rat, IP): 4.328 g/k
LD50 (rat, oral): 36 g/kg | | 貯蔵 | Methionine is sensitive to light and should be stored in a cool, dark
place. | | 純化方法 | Crystallise L-methionine from aqueous EtOH. Also purify it by dissolving ~0.5g of amino acid in ~10mL of hot H2O, filtering, adjusting the pH to 5.8 with 5N HCl, collecting the solid after addition of ~20mL of EtOH. It is recrystallised by dissolving in H2O and adding EtOH. It sublimes at 197-208o/0.3mm with 99.8% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Milne & Peng J Am Chem Soc 79 647 1957, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2125-2152 1961, Beilstein 4 IV 3189.] | | 不和合性 | Methionine is incompatible with strong oxidizing agents. | | 規制状況(Regulatory Status) | Included in the FDA Inactive Ingredients Database (oral tablets).
Included in parenteral preparations (injection solutions; powders
for reconstitution) licensed in the UK. |
|