- Phenylhydrazine
-

- $0.00 / 25KG
-
2023-08-23
- CAS:100-63-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Phenylhydrazine
-

- $1.00 / 1KG
-
2023-08-16
- CAS:100-63-0
- Min. Order: 1KG
- Purity: >99%
- Supply Ability: 50000T
- Phenylhydrazine
-

- $50.00 / 1kg
-
2023-04-27
- CAS:100-63-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons
|
| | Phenylhydrazine Chemical Properties |
| Melting point | 18-21 °C (lit.) | | Boiling point | 238-241 °C (lit.) | | density | 1.098 g/mL at 25 °C (lit.) | | vapor density | 4.3 (vs air) | | vapor pressure | <0.1 mm Hg ( 20 °C) | | refractive index | n20/D 1.607(lit.) | | Fp | 192 °F | | storage temp. | Store below +30°C. | | solubility | Soluble in dilute acids. | | form | Powder | | pka | 8.79(at 15℃) | | color | White to slightly blue or light beige | | explosive limit | 1.1%(V) | | Water Solubility | 145 g/L (20 ºC) | | Sensitive | Air & Light Sensitive | | Merck | 14,7293 | | BRN | 606080 | | Exposure limits | TLV-TWA skin 0.1 ppm (0.44 mg/m3)
(ACGIH), 5 ppm (22 mg/m3) (OSHA);
STEL 10 ppm (44 mg/m3) (OSHA); carcinogenicity: A2-Suspected Human Carcinogen
(ACGIH), Carcinogen (NIOSH).
. | | Dielectric constant | 7.2(23℃) | | Stability: | Stable, but may decompose in sunlight. May be air or light sensitive. Incompatible with strong oxidizing agents, metal oxides. | | CAS DataBase Reference | 100-63-0(CAS DataBase Reference) | | NIST Chemistry Reference | Hydrazine, phenyl-(100-63-0) | | EPA Substance Registry System | Phenylhydrazine (100-63-0) |
| | Phenylhydrazine Usage And Synthesis |
| Hydrazine derivative | Phenylhydrazine is also known as the hydrazinobenzene. It was first successfully synthesized by the German organic chemist Hermann-Emil-Fischer in 1875 and is the first synthesized hydrazine derivatives. At room temperature, it is a pale yellow crystalline or oily liquid while at low temperature it appears as monoclinic prismatic crystals. It can be easily subject to oxidization in the air and exhibit dark brown or dark red.

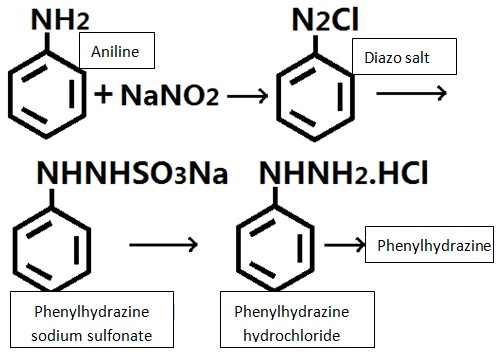
Phenylhydrazine is one of the hydrazine derivatives and is often abbreviated as PhNHNH2. It is slightly soluble in water and alkali solution and can be dissolved in dilute acid. It is miscible with ethanol, ethyl ether, chloroform and benzene. Common method of its preparation is through the reaction between aniline and sodium nitrite under the action of hydrochloric acid for generation of diazonium salt which is then reduced by sulfite/sodium reduction for obtaining it. Acid precipitation can generate phenylhydrazine hydrochloride with neutralization generating phenylhydrazine.
Phenylhydrazine can be applied to making dyes, drugs, and being used as developer. It is also an important reagent for identification of carbonyl group and can be used for identifying aldehydes, ketones and carbohydrates. It can react with benzaldehyde to generate phenylhydrazine. We can use the hydrazone generated through phenylhydrazine or 2, 4-dinitrophenylhydrazine to identify aldehydes and ketones, It can trigger Fischer indole synthesis upon reaction with aldehydes and ketones (first found by the Emil Hermann Fischer in 1883, the reaction is using phenylhydrazine and aldehyde, ketone for heating and rearrangement under acid-catalysis for the elimination of one molecule of ammonia to give 2-or 3-substituted indole.) to give indole ring-class compound. | | Hermann Emil Fischer | Phenylhydrazine (Hydrazinobenzene) is the chemical compound characterized by Hermann Emil Fischer in 1895. Fischer is a German organic chemist. He used phenylhydrazine to react with carbohydrate and generate hydrazone and osazone and form a crystalline compound which is easy for purification. Then it is decomposed into pure sugar. This is a powerful means for studying carbohydrate structures and synthesis of a variety of sugars and enables the theoretic clarification of the structure of glucose and summarizing of the general stereoisomerism phenomenon of saccharides and further using of Fischer projection for describing it. He has also demonstrated that the caffeine, theophylline, uric acid all belongs to purine derivatives and also had successfully synthesized purine. He also had opened up the study of proteins and had demonstrated that amino acids forms polypeptide via peptide bond and successfully synthesized polypeptides. In 1902, Fischer, because of the synthesis of purine and carbohydrate, was awarded for the Nobel Prize in Chemistry.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| | Phenylhydrazine: preparation and application | Phenylhydrazine is the first synthetic hydrazine derivatives which can often be used as intermediates of organic dyes, pharmaceuticals and pesticide. It can also be used as organic intermediates for synthesis of pyrazoline, triazole, and indole; It can also be used as dye intermediates like disazo dye intermediates such as 1-phenyl-3-methyl-5-pyrazolone and so on; It can also be used as pharmaceutical intermediates for preparation of antipyretic, analgesic, anti-inflammatory drugs such as antipyrine and aminopyrine, etc; it can also be used as photography drugs (photosensitive dye); phenylhydrazine is also the raw material for the production of pesticides "imputed phosphorus"; phenylhydrazine is also an important kind of carbonyl group identification reagents used for identifying aldehydes, ketones and carbohydrates.
Phenylhydrazine is toxic. After inhalation, due to hemolysis, it can cause anemia and headache and can even cause jaundice in severe cases. It can also have stimulating effect on the eyes such as causing corneal disorders. Rat-oral LD50:188 mg/kg. Workplace: maximum allowable concentration: 0.44mg/m3.
Lab often uses aniline as raw materials for reaction with sodium nitrite in hydrochloric acid for generating diazonium salt and then reacted with sulfur dioxide and sulfurous acid for reduction to produce phenylhydrazine sodium sulfonate and further salting via hydrochloric acid to form phenylhydrazine hydrochloride. After neutralization and deacidification, we can obtain phenylhydrazine.
| | Chemical Properties | Phenylhydrazine is pale yellow oily liquid with the melting point being 19.8 ℃ and boiling point being 173.5 ℃/13.33kPa, n20D being 1.608 and the relative density being 1.0978 (20 ℃). It is slightly soluble in water and petroleum ether and soluble in alcohol, ether, chloroform and benzene. It can be evaporated together with steam. It is also easily to get dark in the air. Phenylhydrazine hydrochloride [59-88-1] is solid with the m.p. being 250~254 ℃ (decomposition).
| | Uses | In the field of pesticide production, phenylhydrazine can be used for synthesizing the intermediate 1-phenyl-semicarbazide of the organophosphorus pesticides, triazophos as well as the intermediate 1-phenyl-3, 6-hydroxy pyridazine of pyridaphenthione. It is also the intermediates of the novel bactericidal varieties, famoxadone and fenamidone. In addition, hydrazine benzene, as a raw material for organic synthesis, can also be used as the intermediates of dye and pharmaceutical industry as well as being used as an analytical reagent.
The product is the intermediate of dye, pharmaceuticals and pesticides. It can be used for the production of Naphthol AS-G, drugs such as antipyrine and so on. Dissolving the phenylhydrazine in 95% ethanol and following by addition of benzaldehyde and reflux for 1 h can obtain the phenylhydrazone. Phenylhydrazone can also be used as the intermediate of organic synthesis. Phenylhydrazine is also used as an analytical reagent.
As a weak base, it can be used to precipitate lead, chromium and other trivalent, tetravalent element in the form of hydroxide. It can also be used for photometric determination of aluminum, chromium, copper, molybdenum, titanium, and zirconium. It can also be used for verification of gold, iridium, molybdenum, palladium, platinum, and silver. It can be used as the reducing agent for colorimetric assay of phosphorus acid. It can also react with aldehydes, ketones and sugars to get phenylhydrazone with different melting points according which we will be able to identify these substances.
| | Production method | It can be produced from aniline which undergoes diazotization, reduction, acid precipitation to obtain phenylhydrazine hydrochloride and then further neutralization to obtain phenylhydrazine.
The preparation method is based on aniline which successively undergoes diazotization, reduction, acid precipitation, and neutralization to derive it.
Diazotization: add a certain amount of water, 30% hydrochloric acid and aniline to the reaction pot, cooled to 2 ℃, control the temperature at around 0~5 ℃ and add drop wise of solution of sodium nitrite to until the starch iodide test solution turns blue, stir for 30 mins for completion of the reaction and obtain the diazonium solution.
Reduction: add water, sodium bisulfite and 30% alkaline solution to the reduction kettle, heated to 80 ℃, maintain the temperature in 80~85 ℃ and pH = 6.2~6.7, within 20min trickle add the above diazo solution, stirring was continued for 1.5 h, then add zinc powder, diatomaceous earth, stirred filtrate with the filtrate suck into the acid precipitation tank.
Acid precipitation: to the above filtrate add 30% hydrochloric for acid precipitation at 70 ℃, the temperature is maintained in 85~90 ℃, stir for 10min and cool to below 20 ℃, suck, filtration to yield phenylhydrazine hydrochloride.
Neutralization: add the alkaline solution to the reaction pot, stir and simultaneously add the above phenylhydrazine hydrochloride and an appropriate amount of water, heated to 50 ℃, stirred at 50~60 ℃ for 1 h, allowed to stand for more than 8h, separate out the lower aqueous solution, It was the top oil that was phenylhydrazine with the content of about 80% and the total yield of 83.5%.
| | Mutagenicity and Toxicity of Carcinogenic | Phenylhydrazine is toxic by single exposure via the oral route (LD50 80–188 mg/kg body weight) and is expected to be toxic by the inhalation and dermal routes (data from these routes of exposure are less clear). Phenylhydrazine has potential for skin and eye irritation,and there is evidence that it has skin-sensitizing properties in humans. Exposure to phenylhydrazine may cause damage to red blood cells, potentially resulting in anaemia and consequential secondary involvement of other tissues, such as the spleen and liver. Phenylhydrazine is mutagenic in vitro, and there is some evidence to indicate that it may express genotoxic activity in vivo. The substance is clearly carcinogenic in mice following oral dosing, inducing tumours of the vascular system. The mechanism for tumour formation is unclear, but a genotoxic component cannot be excluded. Hence, it is not considered possible to reliably identify a level of exposure at which there will be no risk of carcinogenic or genotoxic effects. | | environmental effects | Phenylhydrazine is degraded photochemically and autoxidizes in water. It is readily biodegradable, and this is expected to be the major route of breakdown in the environment. There is minimal sorption to particulates.
Phenylhydrazine is toxic to aquatic organisms, with the lowest reported no-observed-effect concentration (NOEC) in standard acute fish tests at 0.01 mg/litre; fish are generally more sensitive than either daphnids or bacteria. A NOEC of 0.49 :g/litre has been reported for embryo-larval stages of the zebra fish (Brachydanio rerio). | | Flammable and hazard characteristics | it is combustible upon fire with heating emitting toxic nitrogen compound gas
| | Storage characteristics | Treasury: ventilation, low-temperature and dry; store it separately from oxidants, acids and food additives
| | Extinguishing agent | water, spray, foam, CO2, sand
| | Professional Standards | TLV-TWA 0.1 PPM; TWA 5 PPM (22 mg/cubic meter); STEL 2 mg/m3
| | Chemical Properties | Phenylhydrazine is a colorless to pale yellow liquid or solid with a weak aromatic odor. It is soluble in water (values ranging from 145 to 837 g/litre at 24 °C have been reported) and is miscible with alcohol, ether, chloroform, benzene, and acetone. The conversion factor for phenylhydrazine is 1 ppm = 4.5 mg/m3 (at 20 °C, 101 kPa). | | Physical properties | Yellow monoclinic crystals with a faint, aromatic odor. Turns red-brown on exposure to air. | | Uses | Phenylhydrazine is used in the manufactureof dyes and pharmaceuticals; as a stabilizerfor explosive; as a reagent for aldehydes,ketones, and sugars in chemical analysis; andin organic synthesis. | | Uses | Phenylhydrazine, as hydrochloride solution plus sodium acetate, reacts with polyhydroxy aldehydes or ketones yielding osazones or diphenylhydrazones, yellow solids, of definite melting point and utilized in identification of sugars, e.g., phenyl-d-glucosazone, CH2OH (CHOH)3C: (NNHC6H5)CH:(NNHC6H5) plus aniline C6H5NH2 plus NH3. | | Uses | An aryl hydrazine used in the preparation of various dyes and pharmaceutical compounds. It is used in the investigation of oligosaccharides as well as the structure of photosystem II. | | Definition | ChEBI: A phenylhydrazine that is the monophenyl derivative of hydrazine. | | Synthesis Reference(s) | Synthesis, p. 738, 1975 DOI: 10.1055/s-1975-23917 | | General Description | Pale yellow crystals. Melting point 66°F. Becomes an oily liquid. Toxic by ingestion, inhalation and skin absorption. Flash point 192°F. Autoignition temperature 345°F. Soluble in alcohol. | | Air & Water Reactions | When exposed to air becomes red-brown. Slightly denser than water and slightly soluble in water. | | Reactivity Profile | Phenylhydrazine may ignite spontaneously when in contact with oxidants such as hydrogen peroxide or nitric acid, oxides of iron or copper, or manganese, lead, copper or their alloys. Will not polymerize [USCG, 1999]. | | Health Hazard | Phenylhydrazine is a moderate to highlytoxic compound and a carcinogen. Acutetoxic symptoms include hematuria, changesin liver and kidney, vomiting, convulsions,and respiratory arrest. Additional symptomsare lowering of body temperature and fallin blood pressure. Chronic exposure to thiscompound caused hemolytic anemia andsignificant body weight loss in rats. Anoral administration of 175 mg/kg was lethalto mice. An oral LD50 value in rat is188 mg/kg
Phenylhydrazine caused adverse repro ductive effect when dosed intraperitoneallyin pregnant mice. It caused jaundice, anemia,and behavioral deficit in offspring
Oral or subcutaneous administration ofphenylhydrazine or its hydrochloride produced lung and liver tumors in experimental animals. ACGIH lists this compoundas a suspected human carcinogen. The evidence of carcinogenicity of this compoundin human is inadequate. | | Fire Hazard | Combustible liquid; flash point (closed cup)
89°C (192°F). It reacts with lead dioxide
and strong oxidizing compounds vigorous to
violently. | | Safety Profile | Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion, subcutaneous, and intravenous routes experimental reproductive effects Mutation data reported. Ingestion or subcutaneous injection can cause hemolysis of red blood cells. Other effects are damage to the spleen, liver, kidneys, and bone marrow. The most common effect of occupational exposure is the development of dermatitis, which, in sensitized persons, may be quite severe. Systemic effects include anemia and general weakness, gastrointestinal dsturbances, and injury to the kidneys. Flammable when exposed to heat, flame, or oxidizers. To fight fire, use alcohol foam. Violent reaction with 2phenylamino-3-phenyloxazirane. Reacts with perchloryl fluoride to form an explosive product. Vigorous reaction with lead(Ⅳ) oxide. Used as a chemical reagent, in organic synthesis, and in the manufacture of dyes and drugs. Dangerous; when heated to decomposition it emits highly toxic fumes of NOx; can react with oxidizing materials | | Potential Exposure | Phenylhydrazine is a widely used reagent in conjunction with sugars, aldehydes, and ketones. In addition, to its use in the synthesis of dyes; pharmaceuticals, such as antipyrin; cryogenin, and pyramidone; and other organic chemicals. The hydrochloride salt is used in the treatment of polycythemia vera. | | Carcinogenicity | Mice given 1mg phenylhydrazine
orally each day, 7 days/week for 42 weeks had an
increased incidence of total lung tumors. The incidence of
malignant tumors also increased. In another study,
mice administered 0.01% of the hydrochloride salt in drinking
water (average of 0.63–0.81 mg/day) over their lifetimes
had an increased incidence of blood vessel tumors. In
contrast, mice given phenylhydrazine orally 5 days/week for
40 weeks at doses of 0.5 mg during the first 5 weeks and
0.25 mg thereafter failed to develop tumors.Higher
doses could not be tested because of the marked anemia
encountered. No leukemias occurred in mice treated with
phenylhydrazine, and pulmonary tumors were infrequent
. Oral administration or intraperitoneal injection
(eight doses, each of 2.9 mg) of phenylhydrazine hydrochloride
in mice failed to increase either pulmonary tumors or
leukemia. The carcinogenic activity of hydrazine
itself has been closely linked to its toxicity, that is, its irritant
effect. The mechanism of action, it is postulated, is
indirect alkylation of DNA, which is also closely connected
to the toxic end point. | | Shipping | UN2572 Phenylhydrazine, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. | | Purification Methods | Purify phenylhydrazine by chromatography, then crystallise it from pet ether (b 60-80o)/*benzene. Store it in the dark under N2 as it turns yellow, then red, on exposure to air. It is best stored as the hydrochloride salt; see below. [Coleman Org Synth Coll Vol I 442 1941, Shaw & Stratton J Chem Soc 5004 1962, Beilstein 15 IV 50.] | | Incompatibilities | Phenylhydrazine is very reactive with carbonyl compounds, strong oxidizers; strong bases; alkali metals; ammonia, lead dioxide (violent). Attacks copper salts, nickel, and chromates. | | Waste Disposal | Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device. |
| | Phenylhydrazine Preparation Products And Raw materials |
| Raw materials | Hydrochloric acid-->Sodium nitrite-->Aniline-->Sodium bisulfite-->Potassium iodide-->Celite-->Sodium metabisulfite | | Preparation Products | SULFAPHENAZOLE-->Triazophos E.C.-->3,5-DIMETHYL-1-PHENYL-1H-PYRAZOLE-4-CARBALDEHYDE-->2-Phenyl-1H-indole-1-acetic acid ,97%-->5-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-4-CARBALDEHYDE-->ethyl 2-(2-phenyl-1H-indol-1-yl)acetate-->2-Phenylindole-->4-BROMO-5-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-->Clofentezine-->1-Methyl-2-phenylindole-3-carboxaldehyde-->5-AMINO-1-PHENYLPYRAZOLE-->1,5-Diphenylcarbazide-->ETHYL METHANESULFONYLACETATE-->2,3,5-Triphenyltetrazolium chloride-->1-Methyl-2-phenylindole-->2,2,2-TRIFLUORO-1-(3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOL-4-YL)ETHANONE-->2-(2-NAPHTHYL)-1H-INDOLE-3-CARBALDEHYDE-->1-PHENYL-1H-PYRAZOLE-4-CARBALDEHYDE-->5-CHLORO-1,3-DIPHENYL-1H-PYRAZOLE-4-CARBALDEHYDE-->2-(3-CHLORO-4-FLUOROPHENYL)INDOLE-->4-(1H-INDOL-2-YL)-PHENYLAMINE-->2-(4-FLUOROPHENYL)-1H-INDOLE-3-CARBALDEHYDE-->BISPYRAZOLONE-->5-AMINO-4-CARBETHOXY-1-PHENYLPYRAZOLE-->2-(4-ETHOXY-PHENYL)-1H-INDOLE-->5-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-->1-PHENYLPYRAZOLE-->1,2,3,9-Tetrahydro-4(H)-carbazol-4-one-->2-Phenyl-2-imidazoline-->5-AMINO-1-PHENYLPYRAZOLE-4-CARBONITRILE-->5-METHYL-1-PHENYL-1H-PYRAZOLE-4-CARBOXYLIC ACID-->(5-METHYL-1-PHENYL-1H-PYRAZOL-4-YL)METHANOL-->6-(4-FLUOROPHENYL)INDOLE-->2-(4-BROMO-PHENYL)-1H-INDOLE-->ETHYL 5-METHYL-1-PHENYL-1H-PYRAZOLE-4-CARBOXYLATE-->2-(4-CHLOROPHENYL)INDOLE-->3-METHYL-1-PHENYLPYRAZOLE-->1,2,3,4-Tetrahydro-9-methylcarbazol-4-one-->Phenidone-->Pyrazinamide |
|