- Paliperidone
-

- $0.00 / 1Kg/Bag
-
2024-04-22
- CAS:144598-75-4
- Min. Order: 1Kg/Bag
- Purity: 0.99
- Supply Ability: 20 tons
- Paliperidone
-

- $65.00 / 1kg
-
2024-01-02
- CAS:144598-75-4
- Min. Order: 10kg
- Purity: 0.99
- Supply Ability: 20tons
- Paliperidone
-

- $0.00 / 1KG
-
2023-11-27
- CAS:144598-75-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 TONS
|
| Product Name: | Paliperidone | | Synonyms: | Paliperidone (Invega);3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-Methyl-;Risperidone EP IMpurity C;Paliperidone
9-Hydroxyrisperidone;9-Hydroxyrisperidone solution;Racemic Paliperidone;4h-pyrido(2,1-a)pyrimidin-4-one,6,7,8,9-tetrahydro-3-(2-(4-(6-fluro-1,2-benzis;oxazol-3-yl)-1-piperidinyl)ethyl)-9-hydroxy-2-methyl- | | CAS: | 144598-75-4 | | MF: | C23H27FN4O3 | | MW: | 426.48 | | EINECS: | 620-493-1 | | Product Categories: | Heterocycles;Other APIs;Invega, INVEGA Sustenna, 9-hydroxyrisperidone;Intermediates & Fine Chemicals;Metabolites & Impurities;Pharmaceuticals;Various Metabolites and Impurities;144598-75-4 | | Mol File: | 144598-75-4.mol | 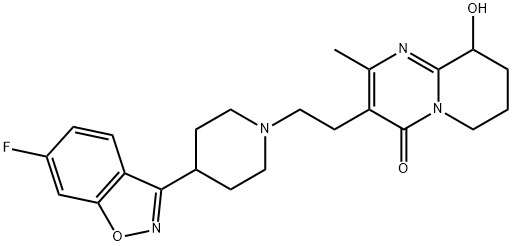 |
| | Paliperidone Chemical Properties |
| Melting point | 158-160°C | | Boiling point | 612.3±65.0 °C(Predicted) | | density | 1.45±0.1 g/cm3(Predicted) | | Fp | 9℃ | | storage temp. | 2-8°C | | solubility | DMSO: soluble2mg/mL, clear (warmed) | | pka | 13.00±0.60(Predicted) | | form | powder | | color | white to brown | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. | | InChI | InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3 | | InChIKey | PMXMIIMHBWHSKN-UHFFFAOYSA-N | | SMILES | C12C(O)CCCN1C(=O)C(CCN1CCC(C3C4=C(ON=3)C=C(F)C=C4)CC1)=C(C)N=2 | | CAS DataBase Reference | 144598-75-4(CAS DataBase Reference) |
| | Paliperidone Usage And Synthesis |
| Description | Paliperidone, (±)-3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one(Invega), is essentially insoluble in water and is available asextended-release tablets. Paliperidone is delivered at a constantrate using an osmotic drug release device (OsmoticRelease Oral Systems [OROS]). Paliperidone is FDA-approved for schizophrenia aged 12–17. However, the pharmacologic portfolio, extrapolation from adult studies, and the long track record of the parent drug, risperidone in child/adolescent psychiatric (CAP) population might expand its therapeutic potential. | | Metabolism | Paliperidone is the primary active metabolite of risperidone. The mechanism of action is unknown but it is likely to act via a similar pathway to risperidone. It has been proposed that the drug's therapeutic activity in schizophrenia is mediated through a combination of central dopamine Type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism. Paliperidone is also active as an antagonist at alpha 1 and alpha 2 adrenergic receptors and H1 histaminergic receptors, which may explain some of the other effects of the drug. Paliperidone was approved by the FDA for treatment of schizophrenia on December 20, 2006. | | Pharmacology | Paliperidone , 9-hydroxyrisperidone, is an atypical antipsychotic, a serotonin (5HT2A) dopamine (D2) antagonist and the active metabolite of the high-potency risperidone. It has also 5HT7 antagonism that might confer some antidepressant actions. It is advantageous given the osmotic-controlled release oral delivery system technique facilitating once-daily dosing, rapid steady-state, and hence better compliance. Moreover, lack of cytochrome CYP-450 enzyme interactions, more alpha-2 affinity, alleged less propensity for extrapyramidal syndromes, and availability of long-acting injection formulation all render paliperidone a very appealing treatment option in psychoses and in hepatic patients too. | | Uses | This medication is used to treat certain mental/mood disorders (such as schizophrenia, schizoaffective disorder). This medication can decrease hallucinations and help you to think more clearly and positively about yourself, feel less agitated, and take a more active part in everyday life.
Paliperidone belongs to a class of drugs called atypical antipsychotics. It works by helping to restore the balance of certain natural substances in the brain. | | Mechanism of Action |
Paliperidone is the major active metabolite of risperidone. The mechanism of action of paliperidone, as with other drugs having efficacy in schizophrenia, is unknown, but it has been proposed that the drug's therapeutic activity in schizophrenia is mediated through a combination of central dopamine Type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism. | | Description | Paliperidone, the C-9 hydroxylated active metabolite of the antipsychotic agent risperidone, is the newest atypical antipsychotic to join the market following the introductions of olanzapine (ZyprexaTM), risperidone (RisperdalTM), quetiapine(SeroquelTM), and ziprasidone (GeodonTM). Compared to its parent, paliperidone has improved PK properties and a reduced potential for drug interactions. In terms of receptor affinity, the two drugs are equipotent. | | Chemical Properties | Off White to Light Orange Coloured Solid | | Originator | Johnson & Johnson (US) | | Uses | A metabolite of Risperidone, a combined serotonin (5-HT2) and dopamine (D2) receptor antagonist | | Uses | Paliperidone(Invega) is an atypical antipsychotic. Chemically, paliperidone is the primary active metabolite of the older atypical antipsychotic risperidone. It is indicated for the acute and maintenance treatment of schizophrenia | | Definition | ChEBI: 3-{2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one is a member of the class of pyridopyrimidines that is 9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one carrying an additional 2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl group at position 2. It is a member of 1,2-benzoxazoles, a heteroarylpiperidine, an organofluorine compound, a pyridopyrimidine and a secondary alcohol. | | Brand name | Invega | | General Description | Paliperidone, (±)-3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one(Invega), is essentially insoluble in water and is available asextended-release tablets. Paliperidone is delivered at a constantrate using an osmotic drug release device (OsmoticRelease Oral Systems [OROS]). The absolute bioavailabilityof paliperidone is 28%, and studies in healthy subjects on ahigh-fat, high-calorie meal showed an increase in AUC.Paliperidone is 74% bound to plasma proteins. After a single,1-mg dose of C-paliperidone, 59% of the dose was excretedin the urine as unchanged drug, and 32% of the dose was recoveredas metabolites. Most of the drug (80%) is excreted bythe kidneys. Paliperidone is metabolized by dealkylation, hydroxylation,dehydrogenation, and scission of the benzoxazolering. None of these metabolic pathways account for morethan 10% of the dose. The terminal elimination half-life ofpaliperidone is 23 hours. | | Biochem/physiol Actions | Paliperidone is an atypical antipsychotic; active metabolite of risperidone. | | Side effects | The most common adverse events
included tachycardia, QTc prolongation, headache, anxiety, dizziness, tremors,
and insomnia along with the dose-related events of somnolence, orthostatic
hypotension, salivary hypersecretion, and extrapyrimidal disorder. Weight gain
was also observed in 6–9% of patients which may be attributable to paliperidone’s
lower affinity for H1-histaminergic and a1- and a2-adrenergic receptors. Patients
with renal impairment require dose adjustments since elimination of paliperidone
is altered. Paliperidone is contraindicated in patients with a hypersensitivity to
risperidone. Concomitant use of class III antiarrhythmic agents should be avoided
since this may result in additive QT interval prolongation. Also, loss of levodopa
efficacy is expected with this D2 antagonist. | | Synthesis | The synthesis of paliperidone involves the reaction of
2-acetyl-g-butyrolactone with 2-amino-3-benzyloxypyridine in the presence of
phosphoryl chloride. The benzyl protecting group of the intermediate 9-benzyloxy-
3-(2-chloroethyl)-2-methylpyrido[1,2-a]pyrimidin-4-one is then removed by
hydrogenolysis over Pd/C followed by the nucleophilic displacement of the
chlorine with 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole to provide racemic
paliperidone. While paliperidone can be resolved, both enantiomers are
equipotent and interconvert in vivo obviating the need for separation. | | Drug interactions | Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias when given with anti-arrhythmics that
prolong the QT interval.
Antidepressants: increases concentration of tricyclics
(possibly increased risk of ventricular arrhythmias).
Antiepileptics: antagonise anticonvulsant effect
(convulsive threshold lowered); concentration
reduced by carbamazepine.
Antimalarials: avoidance of antipsychotics advised by
manufacturer of artemether/lumefantrine.
Antipsychotics: possible increased risk of ventricular
arrhythmias with risperidone.
Antivirals: concentration possibly increased by ritonavir.
Atomoxetine: increased risk of ventricular
arrhythmias with atomoxetine.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide. | | Metabolism | Paliperidone is the active metabolite of risperidone.
Four metabolic pathways have been identified in vivo,
none of which accounted for more than 6.5% of the
dose: dealkylation, hydroxylation, dehydrogenation,
and benzisoxazole scission.
Following administration
of [14C]-paliperidone, 59% of the dose was excreted
unchanged into urine, indicating that paliperidone is not
extensively metabolised in the liver. Approximately 80% of
the administered radioactivity was recovered in urine and
11% in the faeces. | | storage | +4°C | | References | 1) Beijsterveldt?et al.?(1994),?Regional brain distribution of risperidone and its active metabolite 9-hydroxy-risperidone in the rat; Psychopharmacology,?114?53 DOI:10.1007/BF02245444
2) Leysen?et al. (1994),?Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity; J. Clin. Psychiatry,?55?suppl: 5 PMID: 7520908
3) Schotte?et al.?(1996),?Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding; Psychopharmacology (Berl.),?124?57 DOI:10.1007/BF02245606 |
| | Paliperidone Preparation Products And Raw materials |
|