- Letrozole
-

- $9.90 / 1g
-
2024-04-25
- CAS:112809-51-5
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 100kg
- Letrozole
-

- $25.00 / 1box
-
2024-04-24
- CAS:112809-51-5
- Min. Order: 1box
- Purity: 99%
- Supply Ability: 10000box
Related articles - What is Letrozole?
- Letrozole (Femara?) is a nonsteroidal, third-generation aromatase inhibitor administered orally once daily, which has shown ef....
- Feb 10,2020
|
| Product Name: | Letrozole | | Synonyms: | Letrozole99%;CGS-20267, Femara;letrozolex;Lelrozol;1-[BIS(4-CYANOPHENYL)METHYL]-1,2,4-TRIAZOLE;4,4'-(1H-1,2,4-Triazol-1-ylmethylene)dibenzonitrile;4,4'-(1H-1,2,4-Triazole-1-ylmethylene)bisbenzonitrile;4,4'-[(1H-1,2,4-Triazole-1-yl)methylene]dibenzonitrile | | CAS: | 112809-51-5 | | MF: | C17H11N5 | | MW: | 285.3 | | EINECS: | 675-034-8 | | Product Categories: | Anti-cancer&immunity;Aromatics;Heterocycles;APIs;Antibiotics;ELOXATIN;Pharmaceuticals;API's;Inhibitors;Intermediates & Fine Chemicals;Active Pharmaceutical Ingredients;All Inhibitors;anti-neoplastic;Pharmaceutical material and intermeidates;Anti-Cancer;Letrozole;Antineoplastic;112809-51-5 | | Mol File: | 112809-51-5.mol | 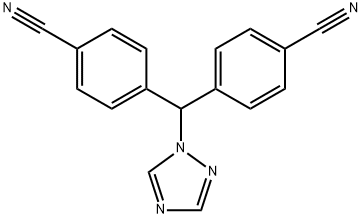 |
| | Letrozole Chemical Properties |
| Melting point | 181-183°C | | Boiling point | 563.5±60.0 °C(Predicted) | | density | 1.21±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMSO: >50mg/mL | | pka | 1.52±0.11(Predicted) | | form | White powder | | color | white to off-white | | Merck | 14,5450 | | InChI | InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | | InChIKey | HPJKCIUCZWXJDR-UHFFFAOYSA-N | | SMILES | C(C1=CC=C(C=C1)C#N)(C1=CC=C(C=C1)C#N)N1C=NC=N1 | | CAS DataBase Reference | 112809-51-5(CAS DataBase Reference) |
| | Letrozole Usage And Synthesis |
| Indications and uses | Letrozole is part of a new generation of highly selective aromatase inhibitors and is an artificially synthesized benzotriazole derivative. Letrozole inhibits aromatase to lower estrogen levels, thus preventing estrogen from stimulating tumor growth. Its in vivo activity is 150-250 times stronger than that of first generation aromatase inhibitor Amarante. As it is highly selective, it will not impact glucocorticoid, mineralocorticoid and thyroid functions; even at high dosages, it will not have any inhibiting effects on adrenal corticosteroid secretion, giving it a high treatment index. Letrozole has no latent toxicity towards any bodily systems and target organs, has no mutagenicity and carcinogenic effects, has minimal toxic side effects, is well-tolerated, and has stronger anticancer effects than other aromatase inhibitors and antiestrogen drugs. Letrozole is suitable for advanced breast cancer postmenopausal patients who have not responded to estrogen-suppressing treatment and for early breast cancer treatment. It is used to treat postmenopausal patients with advanced breast cancer and serves as a second-line treatment to follow unsuccessful antiestrogen treatment. Compared to the current standard Tamoxifen treatment, Letrozole can better prevent the risk of breast cancer recurrence.
| | Pharmacokinetics | Absorption of oral letrozole is rapid and complete and steady state is achieved in 2–6 weeks with administration of letrozole 2.5mg once daily. The major route of elimination of letrozole is via metabolism to a pharmacologically inactive carbinol metabolite. The cytochrome P450 (CYP) 3A4 and CYP2A6 isozymes metabolize letrozole to a pharmacologically inactive carbinol metabolite. Renal excretion of a glucuronide conjugate of the carbinol metabolite of letrozole represents the major route of drug clearance.
| | Side effects | Randomized grouping studies have shown that daily oral ingestion of 2.5mg Letrozole leads to a 33% rate of drug-related negative reactions, a percentage much lower than AG group’s 46%. Negative reactions to Letrozole are mostly mild or moderate, consisting mostly of nausea (2-9%), headache (0-7%), bone pain (4-10%), hot flashes (0-9%) and weight gain (2-8%). Other uncommon side effects include constipation, diarrhea, itching, rash, joint pain, chest pain, abdominal pain, fatigue, insomnia, dizziness, edema, high blood pressure, arrhythmia, thrombosis, dyspnea, vaginal bleeding, etc.
| | Description | Letrozole, also known as Femara, is an orally active aromatase inhibitor that works by competitively inhibiting aromatase. This inhibition prevents the conversion of androgens to estrogen (estrogen stimulates breast tissues and breast cancer reoccurrence) and gonadal steroidogenesis. It can be used for the treatment of breast cancer that is hormonally-responsive or has an unknown receptor status in postmenopausal women. Besides this, Letrozole also has some off-label use such as ovarian stimulation, pretreatment of termination of pregnancy, treatment of gynecomastia, treatment of endometriosis, and promoting spermatogenesis for male patients of nonobstructive azoospermia. | | Chemical Properties | white to light yellow crystal | | Originator | Novartis (Switzerland) | | Uses | A nonsteroidal aromatase inhibitor structurally related to Fadrozole. Antineoplastic | | Uses | Letrozole has been used:
- in organoid growth assay to determine its inhibitory capacity(48)
- to investigate steroid receptor coactivator-1 (SRC-1) mediated endogenous estrogen regulation of hippocampal PSD-95(49)
- to determine its effects on tumor-induced hyperalgesia(50)
- for hormonal manipulation in rats(51)
- to study its effects on lipocalin-2 (Lcn2)(52)
- to determine its effects on mechanical hyperalgesia and aromatase expression(53)
| | Definition | ChEBI: Letrozole is a member of triazoles and a nitrile. It has a role as an antineoplastic agent and an EC 1.14.14.14 (aromatase) inhibitor. | | Manufacturing Process | From 4-bromomethylbenzonitrile and 1H-[1,2,4]triazole was obtained 4-
[1,2,4]triazol-1-ylmethylbenzonitrile. Treatment of that with strong base (tert�BuOK) results in formation of the anion by removal of the relatively acidic
benzyl proton. This anion was condensed with p-fluorobenzinitrile to give
benzhydryl tetrazole (Letrozole) | | Brand name | Femara (Novar tis). | | Therapeutic Function | Antineoplastic | | General Description | Letrozole, 4,4'-(1H-1,2,4-triazol-1-ylmethylene)dibenzonitrile (Femara), is used for most of thesame indications as anastrozole. It reduces concentrations ofestrogens by 75% to 95%, with maximal suppressionachieved within 2 to 3 days. Letrozole is specific for aromataseinhibition, with no additional effects on adrenal corticoidbiosynthesis. CYPs 3A4 and 2A6 are involved in themetabolism of letrozole to the major carbinol metabolite,which is inactive. The loss of the triazole ring, which is involvedin coordination of the heme iron, would explain theloss of activity. Letrozole strongly inhibits CYP2A6 invitro, with moderate inhibition of CYP2C19. The effect ofthis in vitro inhibition on the pharmacokinetics of coadministereddrugs is unknown. Tamoxifen reduces the levels ofletrozole significantly if they are used together, so combinationtreatment with these agents is not recommended. | | Biological Activity | Letrozole is a potent, cell-permeable inhibitor of aromatase (IC50 = 2 nM). It inhibits proliferation of estrogen receptor-positive (ER+) MCF-7 cells when used alone at concentrations ranging from 0.1 to 100 nM and when used at a concentration of 10 nM in combination with testosterone or 4-androstene-3,17-dione. It also reduces matrix metalloproteinase-2 (MMP-2) and MMP-9 levels in MCF-7 cells when used at a concentration of 10 nM. Letrozole (10 μg per day) reduces tumor growth in an MCF-7Ca ovariectomized-mouse xenograft model. Formulations containing letrozole have been used in the treatment of postmenopausal breast cancer. | | Biochem/physiol Actions | Letrozole is a third generation nonsteroidal aromatase inhibitor. It is a competitive inhibitor of the aromatase enzyme system and thus inhibits the conversion of androgens to estrogens. Letrozole inhibits the aromatase enzyme by competitively binding to the heme of the cytochrome P450 subunit of the enzyme, resulting in a reduction of estrogen biosynthesis in all tissues. | | Mechanism of action | Inhibition of arom atase by letrazole is competitive and highly specific , with no effect on enzymes that are responsible for the production of glucocorticosteroids and mineralocorticosteroids. This agent is significantly more effective than tamoxifen in treating horm one-dependent cancer. | | Clinical Use | Femara was launched in France and the UK for second-line treatment of
advanced breast cancer. Letrazole can be synthesized in two steps from 4-
bromomethyl-benzonitrile with 1,2,4-triazole and is a third generation aromatase
inhibitor. It is a highly specific inhibitor of P450arom which prevents the conversion of
androstenedione to estrone. The reduction of plasma estrogen was immediate and
long lasting. This is accomplished with no inhibition of other steroid biosynthesis
making it the most selective aromatase inhibitor tested. Letrazole has remarkable
antitumor activity, is well tolerated and has no toxic side effects. It is 10,000 times
more potent than aminoglutethimide, in vivo, the first well established aromatase
inhibitor. | | storage | Store at +4°C | | References | https://www.drugbank.ca/drugs/DB01006
https://en.wikipedia.org/wiki/Letrozole |
| | Letrozole Preparation Products And Raw materials |
|