- Ammonium sulfate
-

- $0.00 / 25Kg/Bag
-
2024-04-24
- CAS:7783-20-2
- Min. Order: 1000KG
- Purity: 99
- Supply Ability: 10000
- Ammonium sulfate
-

- $300.00 / 10ton
-
2024-04-24
- CAS:7783-20-2
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 2000tons
- Ammonium sulfate
-
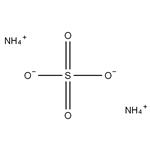
- $8.00 / 1KG
-
2024-04-08
- CAS:7783-20-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
Related articles - Synthesis of Ammonium sulfate
- Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia. A high-me....
- Mar 31,2022
- Uses of Ammonium sulfate
- Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia,it is wide....
- Feb 21,2022
- What is Ammonium Sulfate?
- Ammonium sulfate is used most commonly as an artificial fertilizer for alkaline soils. When introduced into damp soil, an ammo....
- Aug 23,2021
|
| | Ammonium sulfate Chemical Properties |
| Melting point | >280 °C (dec.) (lit.) | | density | 1.77 g/mL at 25 °C (lit.) | | vapor pressure | <1 Pa (25 °C) | | refractive index | n20/D 1.396 | | Fp | 26 °C | | storage temp. | room temp | | solubility | H2O: 1 M at 20 °C, clear, colorless | | form | Solid | | Specific Gravity | 1.769 | | color | Yellow to orange | | PH | 5.0-6.0 (25℃, 1M in H2O) | | Odor | Slight odor of ammonia | | PH Range | 5 - 6 | | Water Solubility | 77 g/100 mL (25 ºC) | | λmax | λ: 260 nm Amax: ≤0.037
λ: 280 nm Amax: ≤0.030 | | Merck | 14,555 | | Stability: | Stable. Contact with strong oxidizers may cause fire or explosion. Incompatible with strong bases. | | InChIKey | BFNBIHQBYMNNAN-UHFFFAOYSA-N | | LogP | -5.1 at 25℃ | | CAS DataBase Reference | 7783-20-2(CAS DataBase Reference) | | EPA Substance Registry System | Ammonium sulfate (7783-20-2) |
| | Ammonium sulfate Usage And Synthesis |
| description | Ammonium sulfate (AS) is the earliest production and use of nitrogen fertilizer. It is usually used as a standard nitrogen fertilizer, nitrogen content is between 20% to 30%. It is a very important fertilizer for any kind of soil that's high in pH and needs a little bit of sulfates to work against the high calcium or the high pH. The nice thing about the ammonium sulfate is that the nitrogen in it is a little bit slower releasing so it lasts throughout the growing season better than the nitrate forms of nitrogen.
In the 1960s, Ammonium sulfate is the main variety of nitrogen fertilizer, but also is a major source to provide crop nutrients sulfur. Firstly ammonia and sulfuric acid was neutralized to obtain, but later increasing proportion of by-product ammonium sulfate, and now Ammonium sulfate is actually produced as a by-product in many industrial processes including the manufacturing of steel, coking industry, caprolactam, sulfuric acid tail gas desulfurization, desulfurization of power plant, acrylonitrile and methyl methacrylate, zinc oxide and some polyester compounds.
Pure product of ammonium sulfate is white crystals, heated to 100 ℃ , began to be decomposed into ammonia and ammonium bisulfate, a by-product with a yellowish or gray, small moisture absorption, easy to agglomerate, it is easier to save and easily soluble in water, insoluble ethanol and acetone.
Ammonium sulfate serves as physiological acidic nitrogen fertilizer, is generally more suitable for wheat, corn, rice, cotton, potato, hemp, fruit trees, vegetables and other crops. For soils, the ammonium sulfate is most suitable for neutral soil and alkaline soil, but not suitable for acidic soil. Also used as analytical reagents (precipitating agent, masking agent), in electrochemical analysis, supports electrolyte, microbiological culture media and preparation of ammonium salts.
The above information is edited by the Chemicalbook of Liu Yujie.
| | nitrogenous fertilizer | Ammonium sulfate was the first nitrogenous fertilizer made by the Haber-Bosch process, produced by the reaction of ammonia with sulfuric acid. In contrast with the nitrate salt, it is chemically stable, not highly hygroscopic. It also supplies supplemental sulfur to soils that may be deficient in this element, but this is of minor value when it is used on soils receiving applications of ordinary superphosphate. The disadvantages of the material are its relatively low nitrogen content, which increases storage and transportation costs, and its marked tendency to cause soil acidification, which is greater than that of any other nitrogen fertilizer material.

| | Uses |
- Ammonium sulfate is a typical Nitrogen-based, water-soluble, and fast acting fertilizer, for various soil and crop. It is used largely as an artificial fertilizer for alkaline soils. In the soil the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil , while contributing essential nitrogen for plant growth.
- For the analysis reagents, also for protein precipitation.
- Used as flux, fire retardant in textile fabric industry, as the salting-out agent, osmotic pressure regulating agents in medicine.
- Used as raw materials of hydrogen peroxide , ammonium chloride, ammonium alum and production in chemical industry, as a flux in the welding industry.
- Used as plating bath additives in electroplating industry.
- Used as dough modifier, yeast nutrients in food grade product.
- Ammonium sulfate is also used as an agricultural spray adjuvant for water soluble insecticides, herbicides, and fungicides. There it functions to bind iron and calcium cations that are present in both well water and plant cells.
| | Toxicity | LD50 orally in Rabbit: 2840 mg/kg | | Description | Ammonium sulfate was the first
nitrogenous fertilizer made by the Haber-Bosch process,
produced by the reaction of ammonia with sulfuric acid. In
contrast with the nitrate salt, it is chemically stable, not
highly hygroscopic. It also supplies supplemental sulfur to
soils that may be deficient in this element, but this is of
minor value when it is used on soils receiving applications
of ordinary superphosphate.
The disadvantages of the
material are its relatively low nitrogen content, which
increases storage and transportation costs, and its marked
tendency to cause soil acidification, which is greater than
that of any other nitrogen fertilizer material. | | Chemical Properties | White crystalline powder | | Physical properties | White crystalline solid; orthorhombic crystal; density 1.769 g/cm3 at 20°C; melts between 511 to 515°C (in a closed system): however, in an open system, it melts with decomposition at 280°C; readily dissolves in water (solubility, 70.6 g and 104 g per 100 g water at 0°C and 100°C, respectively); insoluble in acetone, alcohol and ether. | | Occurrence | Ammonium sulfate occurs in trace concentrations in the upper atmosphere. It is widely used as a fertilizer for rice and other crops. It is a source of sulfur for the soil. It is also used as an additive to supply nutrient nitrogen in fermentation processes (e.g., yeast production from molasses). It also is used for fireproofing timber and plastics, and in treatment of hides, and leather production. | | Uses | May be used for the precipitation or fractionation of proteins or for purification of antibodies. Useful for crystallographic analysis of nucleic acids and proteins. | | Uses | Ammonium Sulfate is a dough conditioner, firming agent, and pro-
cessing aid which is readily soluble in water with a solubility of
approximately 70 g in 100 g of water at 0°c. the ph of a 0.1 molar
solution in water is approximately 5.5. it is used in caramel produc-
tion and as a source of nitrogen for yeast fermentation. in bakery
products, up to 0.25 part per 100 parts by weight of flour is used. | | Uses | manufacture of ammonia alum; in the manufacture of H2SO4 to free it from nitrogen oxides; analytical uses; freezing mixtures, flameproofing fabrics and paper; manufacture of viscose silk; tanning, galvanizing iron; in fractionation of proteins. The commercial grade is used as fertilizer. | | Definition | ammonium sulphate: A whiterhombic solid, (NH4)2SO4; r.d. 1.77;decomposes at 235°C. It is very solublein water and insoluble in ethanol.It occurs naturally as the mineralmascagnite. Ammonium sulphatewas formerly manufactured from the‘ammoniacal liquors’ produced duringcoal-gas manufacture but is nowproduced by the direct reaction betweenammonia gas and sulphuricacid. It is decomposed by heating torelease ammonia (and ammoniumhydrogensulphate) and eventuallywater, sulphur dioxide, and ammonia.Vast quantities of ammoniumsulphate are used as fertilizers. | | Production Methods | Ammonium sulfate is a high-tonnage industrial chemical, but frequently may be considered a byproduct as well as intended end-product of manufacture. A significant commercial source of (NH4)2SO4 is its creation as a byproduct in the manufacture of caprolactam, which yields several tons of the compound per ton of caprolactam made. Ammonium sulfate also is a byproduct of coke oven operations where the excess NH3 formed is neutralized with H2SO4 to form (NH4)2SO4. In the Meresburg reaction, natural or byproduct gypsum is reacted with ammonium carbonate: CaSO4·2H2O + (NH4)2CO3 CaCO3 + (NH4)2SO4 +2 H2O The product is stable, free-flowing crystals. As a fertilizer, (NH4)2SO4 has the advantage of adding sulfur to the soil as well as nitrogen. By weight, the compound contains 21% N and 24% S. Ammonium sulfate also is used in electric dry cell batteries, as a soldering liquid, as a fire retardant for fabrics and other products, and as a source of certain ammonium chemicals. | | General Description | White odorless solid. Sinks and dissolves in water. | | Air & Water Reactions | Dissolves in water with evolution of some heat. | | Reactivity Profile | Ammonium sulfate is acidic in aqueous solution. When a little Ammonium sulfate is added to fused potassium nitrite, a vigorous reaction occurs attended by flame [Mellor 2:702 1946-47]. | | Flammability and Explosibility | Not classified | | Agricultural Uses | Ammonium sulphate, (NH4)2SO4, a water-soluble
crystalline salt is a nitrogenous fertilizer containing about
2 1 % nitrogen and 24 % sulphur. It occurs naturally as the
mineral mascagnite and offers many advantages as a
fertilizer, such as low hygroscopicity, good physical
properties, excellent chemical stability, good agronomic
effectiveness and long shelf life.
Ammoniacal nitrogen is fned in the soil in an
exchangeable form until nitrated by nitrifying bacteria.
The ammoniacal nitrogen of ammonium sulphate does
not leach out easily. Ammonium sulphate is an acid
forming fertilizer, and hence used in neutral or alkaline
soils. In its free flowing form, it is directly applied to the
soil or blended with other granular materials.
Ammonium sulphate also supplies sulphur, which is an
essential nutrient for plants.
Ammonium sulphate is a quick-acting fertilizer. It is
resistant to leaching as it gets adsorbed on the soil
colloids, clay and humus, and replaces calcium. This
adsorbed ammonium salt is converted to nitrate by
nitrifying bacteria for use by growing plants.
Ammonium sulphate is produced in different ways,
The major ones are: (i) Production from synthesized
ammonia and sulphuric acid.
(ii) Production of ammonium sulphate fertilizer by the
gypsum process is widely used in many developing
countries. In this process, ammonia is used along with
pulverized calcium sulphate, carbon dioxide and water.
Here ammonia made from nitrogen and hydrogen, reacts
with carbon dioxide gas to produce ammonium
carbonate. Ground gypsum reacts with ammonium
carbonate solution to form ammonium sulphate and
calcium carbonate.
Ammonium sulphate is commonly transported in
polythene or paper bags. It is adsorbed on soil colloids,
clay and humus, replacing calcium. It is more beneficial
than nitrate fertilizers at planting time. This adsorbed
portion is slowly released and in about a month most of
the ammonium sulphate is converted into the nitrate
form, which is used by growing plants.
Since rice crops absorb nitrogen even in the
ammoniacal form, ammonium sulphate fertilizer is used
as a source of nitrogen for rice in the USA and Southeast
Asia. In the USA, ammonium sulphate is also used for
potato scab control.
The main disadvantages of ammonium sulphate are its
acid forming nature, low nitrogen percentage
(21%) and high costs for packaging, storage and
transportation. | | Biochem/physiol Actions | Ammonium sulfate?((NH4)2SO4) is mainly used as a soil fertilizer. It is also used as a wood preservative. This inorganic salt plays a role in flame retardant chemicals. | | Safety Profile | Moderately toxic by
several routes. Human systemic effects by
ingestion: hypermotility, diarrhea, nausea or
vomiting. See also SULFATES.
Incandescent reaction on heating with
potassium chlorate. Reaction with sodmm
hypochlorite gves the unstable explosive
nitrogen trichloride. Incompatible with (K +
NH4NO3), KNO2, (NaK + NH4NO3).
When heated to decomposition it emits very
toxic fumes of NOx, NH3, and SOx. | | Purification Methods | Crystallise it twice from hot water containing 0.2% EDTA to remove metal ions, then finally from distilled water. Dry it in a desiccator for 2 weeks over Mg(ClO4)2. After 3 recrystallisations, ACS grade had Ti, K, Fe, Na at 11, 4.4, 4.4, 3.2 ppm respectively. |
| | Ammonium sulfate Preparation Products And Raw materials |
|