- Rosuvastatin calcium
-

- $50.00 / 1kg
-
2024-04-24
- CAS:147098-20-2
- Min. Order: 1kg
- Purity: 99.10%
- Supply Ability: 50000kg
- Rosuvastatin Calcium
-

- $220.00 / 1Kg/Bag
-
2024-04-22
- CAS:147098-20-2
- Min. Order: 1Kg/Bag
- Purity: EP / GMP
- Supply Ability: 20 tons
- Rosuvastatin calcium
-

- $0.00 / 1KG
-
2024-03-16
- CAS:147098-20-2
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
|
| Product Name: | Rosuvastatin calcium | | Synonyms: | (+)-bis[7-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]-3R,5S-dihydroxy-6(E)-heptenoic acid]calcium salt;calcium (3R,5S,E)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethan-3-ylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoate;ZD4522;(3R,5S,6E)-7-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[N-methyl(n-methylsulfonyl)amino]-5-pyrimidinyl]-3,5-dihydroxy-6-Hep;Rosuvastatin (as Ca salt);ROSUVASTATIN, CALCIUM SALT;ROSUVASTATIN CALCIUM;Calcium (3R,5S,E)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5-y | | CAS: | 147098-20-2 | | MF: | C22H30CaFN3O6S | | MW: | 523.63 | | EINECS: | 627-028-1 | | Product Categories: | Inhibitors;Active Pharmaceutical Ingredients;Aromatics;Chiral Reagents;Sulfur & Selenium Compounds;HMG-COA reductase, Hypolipidemic drugs;rosuvastatin calcium R;API;Intermediates & Fine Chemicals;Pharmaceuticals;Statins’Anti-Lipidemia Agents;Rosuvastatin;DERMATOP;147098-20-2;Coronavirus | | Mol File: | 147098-20-2.mol | 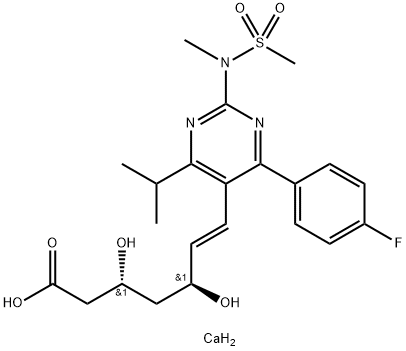 |
| | Rosuvastatin calcium Chemical Properties |
| Melting point | 122°C | | alpha | D24 +14.8° (c = 1.012 in 50% methanol) | | storage temp. | 2-8°C | | solubility | DMSO : 25 mg/mL (49.94 mM; Need ultrasonic)H2O : 1 mg/mL (2.00 mM; Need ultrasonic) | | form | powder | | color | white to beige | | optical activity | [α]/D +12 to +18°, c = 1 in methanol: water (1:1) | | λmax | 243nm(Phosphate buffer sol.)(lit.) | | Merck | 14,8270 | | InChIKey | JSVIQRTVYKCCOM-LMRKSJJTNA-N | | SMILES | C(/C1=C(N=C(N(C)S(=O)(=O)C)N=C1C1C=CC(F)=CC=1)C(C)C)=C\[C@@H](O)C[C@@H](O)CC(=O)O.[Ca] |&1:24,27,r| | | CAS DataBase Reference | 147098-20-2(CAS DataBase Reference) |
| RTECS | MJ9675070 | | HS Code | 29350090 |
| | Rosuvastatin calcium Usage And Synthesis |
| Anti-hyperlipidemia drugs | Rosuvastatin calcium is an anti-hyperlipidemia drug which belongs to the inhibitor of HMG-CoA reductase successfully developed by the British AstraZeneca company. It is suitable for the treatment of various lipid abnormalities, including hypercholesterolemia, mixed lipid qualitative abnormalities and simple hypertriglyceridemia. Rosuvastatin calcium is listed as the strongest and the most comprehensive statins drugs which has entered into market for lipid-lowering and adjustment. Compared with atorvastatin which is currently recognized with the world's best efficacy, it has a better effect of lowering the level of LDL cholesterol and increasing the level of high-density lipoprotein. Moreover, it has a better tolerance, lower side effects and unique pharmacokinetic characteristics with the half-life about 20h. It only needs to be taken once a day.
| | Pharmacological effects | Rosuvastatin is a selective inhibitor of HMG-CoA reductase. HMG-CoA reductase inhibitor is the rate-limiting enzyme of the transition hydroxy-3-methylglutaryl coenzyme A to valerate A (The precursor of cholesterol). The main site of action of rosuvastatin is liver-the target organs of cholesterol-lowering. Rosuvastatin increases the number of hepatic LDL receptors on the cell surface, promoting the absorption and catabolism of LDL, inhibits hepatic synthesis of VLDL, thereby reducing the total number of VLDL and LDL particles.
For patients of homozygous and heterozygous familial hypercholesterolemia, non-familial hypercholesterolemia, and mixed dyslipidemia, Rosuvastatin can lower the total cholesterol, LDL-C levels, ApoB levels, non-HDL-C levels. Rosuvastatin also reduces the level of TG and increase the level of HDL-C. For patients of pure hypertriglyceridemia, rosuvastatin can lower total cholesterol, LDL-C, VLDL-C, ApoB, non-HDL-C, TG levels and increase the HDL-C levels.
Based on the general safety of pharmacology, repeated dose toxicity, potential genetic toxicity and the clinical information of carcinogenicity, no special toxicity was found for rosuvastatin on the human body. For studies on rat before and after birth, Rosuvastatin has obvious reproductive toxicity. It reduces the size, weight of the litter and birth rate. Treating female rats with a toxic dose which causes several times higher systemic exposure levels than the therapeutic exposure can enable the observation of these phenomena.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| | Pharmacokinetics | The drug can be absorbed in great amount by liver after oral administration with the volume of distribution at 134 L. After 3~5 hours, plasma concentration reaches the peak. The absolute bioavailability is around 20%. Plasma protein binding rate (mainly albumin) is about 90%. About 90% of the total dose of rosuvastatin is excreted by feces as prototype (including the absorption of the active substance and unabsorbed), with the rest excreted through the urine. 5% rosuvastatin in urine is in the form of prototype. Half-life of plasma elimination is about 19 hours. Elimination half-life doesn’t increase with increased dose. The geometric mean of plasma clearance rate is about 50L/hour (coefficient of variation 21.7%). Same as other HMG-CoA reductase inhibitors, the uptake of rosuvastatin by liver involves the participation of membrane transporter OATP-C. The transporter is important in the liver for the clearance of rosuvastatin.
| | Drug Interactions | 1. Cyclosporine: when this product is used in combination with cyclosporine, the AUC of rosuvastatin is averagely 7 times higher than that observed in healthy volunteers (compared with the same dose using this service). Combination usage does not affect the plasma concentrations of cyclosporine.
2. Vitamin K antagonists: same as other HMG-CoA reductase inhibitor, for patients of simultaneously usage of vitamin K antagonists (such as warfarin), at the beginning or gradually increasing the dose of this product may cause the increase of INR (international normalized ratio). Stop using the product or gradually decreasing the dose can lead to decrease in INR. In this case, appropriate INR testing is needed.
3. Combination of gemfibrozil, fenofibrate, other fibrates and lipid-lowering doses (≥1g/day) of niacin with HMG-CoA reductase inhibitors can increase the risk of myopathy, which may be due to that they can cause myopathy when given alone.
4. Antacids: Mixing the product and one antacid containing aluminum hydroxide and magnesium for suspension can reduce the concentration of statin rosuvastatin plasma by 50%. Taking antacids two hours after taking this can further reduce the impact. The clinical significance of this drug interaction has not been studied.
5. Erythromycin: Combination with erythromycin can cause rosuvastatin AUC (0~t) decrease by 20% and Cmax decrease by 30%. This interaction may be due to the gastrointestinal motility caused by increase of erythromycin.
6. Oral administration of contraceptives/hormone replacement therapy (HRT): while using this drug together with oral contraceptives can cause the increase of ethinyl estradiol and norgestrel AUC by 26% and 34%, respectively. When choosing the dose of oral contraceptives, the patients should consider the increase of the plasma concentration of these drugs. Yet there are no pharmacokinetic data on taking rosuvastatin and HRT together. Therefore, we cannot exclude the presence of similar interactions. However, in clinical trials, this kind of combination is widely applied and can be well tolerated by patients.
| | Synthesis pathways | 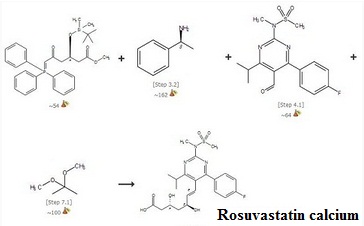
Figure 1 Synthesis pathways of Rosuvastatin calcium | | Indications | Primary Hypercholesterolemia: Usual dose of rosuvastatin calcium (10 mg) can significantly reduce LDLC levels in patients of primary hypercholesterolemia, and has a better efficacy compared with atorvastatin calcium (10mg). At the end of the experiment, TC, TG levels drop even more. Moreover, 5 mg of rosuvastatin calcium within the same eight weeks of treatment has a better effect on reducing LDLC level compared with 10 mg atorvastatin calcium. The difference is statistically significant, suggesting that the lipid-lowering effect of rosuvastatin calcium is better than that of atorvastatin calcium. Our experimental results are also consistent with the other previous assessment (STELLAR, MERCURY) about efficacy studies on statin drugs. Rosuvastatin calcium has a stronger LDLC-lowering effect than the other statins drugs (atorvastatin, simvastatin and pravastatin), allowing more patients to achieve recommended treatment goals.
Elderly coronary heart disease combined hyperlipidemia: Rosuvastatin calcium is capable of competitively inhibiting liver methylglutaryl coenzyme A reductase, and further exerts its good effect on reducing the fat. It has a long half-life. The gender and age of patients have no significant effect on the metabolism of drugs. The drug has only a few of adverse reactions. Meanwhile, the drug can also effectively promote the transport of low-density lipoprotein into cells, thus improving the cleaning effect of LDL, and playing a role in effectively lowering LDL. In addition, rosuvastatin calcium may also inhibit the aggregation of platelet, reduce the body's inflammatory response and protect their endothelial cells, effectively stabilize the plaque of patients suffering coronary artery disease to reduce its impact on the patient. The patients of experimental group in our hospital were treated with rosuvastatin calcium, which gives a significantly better lipid-lowering effect than patients in the control group, P <0.05, the difference was statistically significant. This further illustrates the usage of rosuvastatin calcium for treatment of elderly coronary heart disease combined hyperlipidemia has a good effect which can improve efficacy, reduce the blood fat with high clinical significance.
| | Safety | In terms of safety issue, the incidence of adverse cases for rosuvastatin calcium was similar to that of other related drugs. Adverse cases include elevated ALT, increased GGT and nausea.
| | Side effects | 1. Adverse reactions of this product are generally mild and transient such as common headache, dizziness, constipation, nausea, abdominal pain, muscle pain and weakness.
2. For observed patients, the increase of creatine kinase (CK) levels is dose-related; the majority of cases were mild, asymptomatic and transient. Stop the treatment once the CK levels elevate (> 5 × ULN).
3. Impact on the liver: for a small number of patients, it was observed for a drug dose-related increase in transaminases; the majority of cases were mild, asymptomatic and transient.
| | Uses | For making lipid-lowering drugs.
| | Description | Rosuvastatin, one of the two new statins launched for the treatment of
hypercholesterolemia, has high hepato-selectivity and more potent inhibitory effect
on HMG-CoA reductase than the previously marketed statins. In rat hepatocytes, it
inhibits cholesterol biosynthesis with an IC50 of 1.12 nM, which is ~100-fold higher
potency than pravastatin. Rosuvastatin is synthesized in a 12-step sequence, entailing
the construction of a pyrimidinyl aldehyde intermediate in eight steps and subsequent
introduction of the dihydroxyheptenoate side chain via Wittig reaction with a bketophosphorane
reagent and stereoselective carbonyl reduction of the resultant
enone. Pharmacokinetic properties of rosuvastatin in humans, dosed at 5–80 mg, are
approximately linear with dose. Following oral administration, rosuvastatin is rapidly
absorbed with an oral bioavailability of ~20% and tmax of ~3 h. It has a prolonged
duration of action, with terminal t1/2 of ~20 h, compatible with once-daily dosing.
In humans, rosuvastatin is minimally metabolized through CYP2C9 and CYP2C19,
with little or no metabolism via the CYP3A4. Approximately 90% of the
administered oral dose is eliminated in the feces (92% as the parent compound)
and the rest in the urine. Rosuvastatin is considered a “superstatin” due to its ability,
at well-tolerated doses, to lower LDL cholesterol and triglycerides to a much greater
extent than first generation statins. In patients with hypercholesterolemia, rosuvastatin
treatment at doses of 5 and 10 mg/day over 12-week period resulted in 40–43%
reduction of LDL-cholesterol levels, 12–13% increase in HDL-cholesterol, and 17–
19% reduction in triglycerides. In comparison, the efficacy range of LDL-cholesterol
reductions by atorvastatin (10 mg/day), pravastatin (20 mg/day), and simvastatin
(20 mg/day) was 28–35%. Rosuvastatin is a well-tolerated drug at doses of 1–20 mg and the most common side effects at these doses are headache, myalgia, pain and
pharyngitis, which are consistent with those previously reported for statin therapy. | | Chemical Properties | White to Off-White Crystalline Solid | | Originator | Shinogi (Japan) | | Uses | Rosuvastatin Calcium Salt is a selective, competitive HMG-CoA reductase inhibitor. Antilipemic. | | Uses | antiinflammatory, glucocorticoid | | Uses | antirheumatic | | Definition | ChEBI: An organic calcium salt that is the hemicalcium salt of rosuvastatin. | | Brand name | Crestor (AstraZeneca). | | General Description | Rosuvastatin calcium (ROS) has a molecular mass of 1001.14. ROS is a member of the "statins" group and is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor. | | Biochem/physiol Actions | Rosuvastatin calcium is a competitive inhibitor of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonic acid, the rate-limiting step in cholesterol biosynthesis. Rosuvastatin calcium is antilipemic and is used to reduce plasma cholesterol levels and prevent cardiovascular disease. | | Mechanism of action | Rosuvastatin is a statin medication and a competitive inhibitor of the enzyme HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase, which catalyzes the conversion of HMG-CoA to mevalonate, an early rate-limiting step in cholesterol biosynthesis.24 Rosuvastatin acts primarily in the liver, where decreased hepatic cholesterol concentrations stimulate the upregulation of hepatic low density lipoprotein (LDL) receptors which increases hepatic uptake of LDL. Rosuvastatin also inhibits hepatic synthesis of very low density lipoprotein (VLDL).44 The overall effect is a decrease in plasma LDL and VLDL. | | Synthesis | The synthesis of optically pure rosuvastatin (23) begins from
the Knoevenagel reaction of p-fluorobenzaldehyde (193) with
ethyl isobutylacetate (194) to give unsaturated ketoester 195. Compound 195 was condensed with (S ) -
methylisothiourea and then aromatized in situ using DDQ in
methylene chloride to give pyrimidine 196 in 50% yield.
Pyrimidine sulfide 196 was then oxided by m-CPBA to give
sulfone 197 in 96% yield. Sulfone 197 was reacted with
methylamine in methanol followed by treatment with
methanesulfonyl chloride to give the N -
methanesulfonylamino pyrimidine 198 in 58% yield.
Reduction of ester 198 with DIBAL-H followed by TPAP
oxidation afforded aldehyde 199 in 58% yield. Aldehyde
199 was subjected to Wittig reaction with optically pure
ylide, (3R)-3-(t-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
(200), to give heptenoate
compound 201 in 71% yield. Compound 201 was
deprotected with HF in acetonitrile, and stereoselective
chelation-controlled reduction with Et2BOMe and NaBH4 in
THF-MeOH mixed solvent gave methyl (3R, 5S, 6E)-
dihydroxyheptenoate 202 in 85% yield. Diol 202 was
hydrolyzed with aqueous NaOH to afford the corresponding
sodium salt. Rosuvastatin calcium salt (23) was obtained as
white powder from the sodium salt on treatment with
aqueous CaCl2. 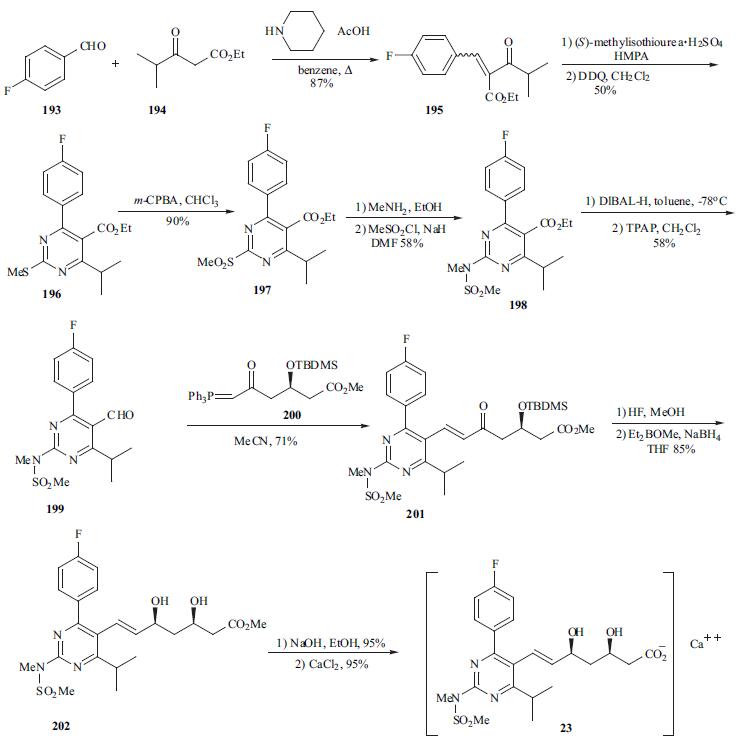
| | storage | Store at +4°C |
| | Rosuvastatin calcium Preparation Products And Raw materials |
|