| | CYCLOBARBITAL Basic information |
| Product Name: | CYCLOBARBITAL | | Synonyms: | 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-(1-cyclohexen-1-yl)-5-ethyl-;5-(1-Cyclohexen-1-yl)-5-ethyl-2,4,6(1H,3H,5H)-pyrimidinetrione;5-(1-cyclohexen-1-yl)-5-ethyl-barbituricaci;5-(1-Cyclohexenyl)-5-ethylbarbituric acid;5-(1-cyclohexenyl)-5-ethylbarbituricacid;5-Ethyl-5-cyclohexenylbarbituric acid;5-ethyl-5-cyclohexenylbarbituricacid;Adorm | | CAS: | 52-31-3 | | MF: | C12H16N2O3 | | MW: | 236.27 | | EINECS: | 200-138-3 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Amines;Heterocycles | | Mol File: | 52-31-3.mol | 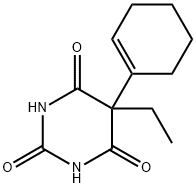 |
| | CYCLOBARBITAL Chemical Properties |
| Melting point | 171-174° | | Boiling point | 378.73°C (rough estimate) | | density | 1.1623 (rough estimate) | | refractive index | 1.5460 (estimate) | | solubility | Chloroform (Slightly), Ethanol (Slightly), Methanol (Slightly) | | form | Solid | | pka | 8.03±0.10(Predicted) | | color | White to Off-White | | Water Solubility | 8.27g/L(25 ºC) |
| RIDADR | 3249 | | HazardClass | 6.1(b) | | PackingGroup | III | | Toxicity | LD50 in mice, rats (mg/kg): 350, 290 i.p. (Hofrichter) |
| | CYCLOBARBITAL Usage And Synthesis |
| Originator | Cyclobarbital,Bayer | | Uses | Cyclobarbital has been used as an anesthetic and sedative. Controlled substance (depressant). | | Definition | ChEBI: Cyclobarbital is a member of barbiturates. | | Manufacturing Process | 772.0 g of δ-1,2-cyclohexenylcyanacetic acid ethyl ester are introduced into astirred and ice cooled solution of 92.0 g of sodium in 1500 ml of absolutealcohol. The sodium δ-1,2-cyclohexenylcyanacetic acid ester formed is thengradually treated without ice cooling with 750.0 g of ethyl iodide. The reactionmixture become warm, sodium iodide separates out and the whole is neutralafter a short time. The sodium iodide is filtered off, the filtrate freed fromalcohol by distillation, the residues taken up in water, siphoned off, dried overcalcium chloride and distilled in vacuum, yields δ-1,2-cyclohexenylethylcyanacetic acid ethyl ester, boil point 125°C. 72.0 g ofsodium are dissolved in 1086.0 g of absolute alcohol and boiled for 3.75 hwith 285.0 g of guanidine sulfate, then 221.0 g of δ-1,2-cyclohexenylethylcyanacetic acid ester are added and boiling is continued for afurther 12 h. The residue remaining after distilling off the alcohol is boiledwith 10 times its weight of dilute sulfuric acid and then δ-1,2-cyclohexenylethylbarbituric acid which separates out is recrystallized from hotwater, melting point 170°C | | Brand name | Phanodorn (Sterling Winthrop). | | Therapeutic Function | Hypnotic | | Safety Profile | Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: pulmonary consolidation. Used as a central nervous system depressant, hypnotic, and sedative. When heated to decomposition it emits toxic fumes of NOx. See also BARBITURATES. |
| | CYCLOBARBITAL Preparation Products And Raw materials |
|