- Everolimus
-
- $0.00 / 1g
-
2024-04-19
- CAS:159351-69-6
- Min. Order: 1g
- Purity: 99%+
- Supply Ability: 100kgs per Month
- Everolimus
-

- $0.00 / 25kg
-
2024-04-12
- CAS:159351-69-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000ton
- Everolimus
-

- $0.00 / 25Kg/Bag
-
2024-04-10
- CAS:159351-69-6
- Min. Order: 2Kg/Bag
- Purity: 0.0099
- Supply Ability: 20 tons
Related articles - What is Everolimus?
- Everolimus is an orally administered, targeted therapy indicated for the treatment of advanced renal cell carcinoma. It inhibi....
- Feb 13,2020
|
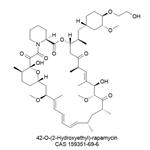
| Product Name: | Everolimus | | Synonyms: | Rapamycin, 42-O-(2-hydroxyethyl)-;Everolimus;Certican;CERTICAN(R);EveroliMus(RAD-001);(1R,9S,12S,15R,16Z,18R,19R,21R,23S,24Z,26Z,28Z,30S,32S,35R)-1,18-dihydroxy-12-[(2R)-1-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-Methoxycyclohexyl]propan-2-yl]-19,30-diMethoxy-15,17,21,23,29,35-hexaMethyl-11,36-dioxa-4-azatricyclo[30.3.1.0^{4,9}]hexatriaconta-16,2;EveroliMus API;23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine, rapamycin deriv | | CAS: | 159351-69-6 | | MF: | C53H83NO14 | | MW: | 958.22 | | EINECS: | 621-003-9 | | Product Categories: | Akt;mTOR;PI3K;Anti-cancer&immunity;PI3K/Akt/mTOR;Inhibitor;Antineoplastic protein kinase inhibitors;mTOR inhibitor;Certican, Zortress, Afinitor;Everolimus;All Inhibitors;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;API;159351-69-6 | | Mol File: | 159351-69-6.mol | 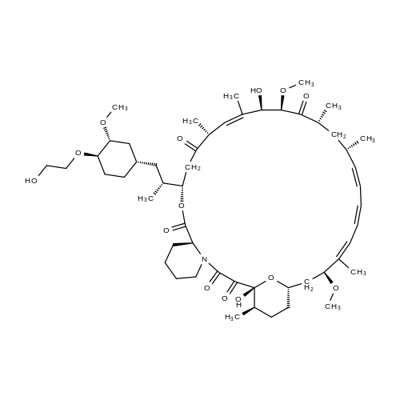 |
| | Everolimus Chemical Properties |
| Melting point | NA | | Boiling point | 998.7±75.0 °C(Predicted) | | density | 1.18±0.1 g/cm3(Predicted) | | Fp | 2℃ | | storage temp. | -20°C | | solubility | Soluble in DMSO (up to 100 mg/ml) or in Ethanol (up to 100 mg/ml). | | form | solid | | pka | 10.40±0.70(Predicted) | | color | White | | Water Solubility | Soluble in dimethysulfoxide,ethanol and chloroform. Slightly soluble in water. | | Stability: | Hygroscopic | | InChIKey | HKVAMNSJSFKALM-GKUWKFKPSA-N |
| | Everolimus Usage And Synthesis |
| Overview | Everolimus is a derivative of Rapamycin(sirolimus), and works similarly to Rapamycin as an mTOR(mammalian target of rapamycin) inhibitor[1-3]. It is currently used as an immunosuppressant to prevent rejection of organ transplants. In a similar fashion to other mTOR inhibitors, Everolimus takes effect solely on the mTORC1 protein and not on the mTORC2 protein[1]. In transplantation medicine, it is marketed under the trade names Zortress(USA) and Certican(Europe)[4]. It has been also used for the treatment of tumors, being marketed as Afinitor(general tumours) and Votubia(tumours as a result of TSC) in oncology[5, 6].
| | Indications | Everolimus is indicated for the treatment of numerous diseases and disorders. It indicated for the treatment of the following cases including both tumors and organ transplantation:
Patients with advance kidney cancer[7];
Postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer(advanced HR+ BC) in combination with exemestane, after failure of treatment with letrozole or anastrozole[8-10];
Adult patients with progressive neuroendocrine tumors of pancreatic origin(PNET) with unresectable, locally advanced or metastatic disease[9, 10];
Adult patients with advanced renal cell carcinoma(RCC) after failure of treatment with sunitinib or sorafenib;
Adult patients with renal angiomyolipoma and tuberous sclerosis complex(TSC), not requiring immediate surgery[11];
Pediatric and adult patients with tuberous sclerosis complex(TSC) for the treatment of subependymal giant cell astrocytoma(SEGA) that requires therapeutic intervention but cannot be curatively resected[12];
Adult and pediatric patients aged 2 years and older with Tuberous Sclerosis Complex(TSC)-associated partial-onset seizures[10];
Preventing the organ rejection during/after renal and liver transplantation[14, 15];
Progressive, well-differentiated non-functional, neuroendocrine tumors[NET] of gastrointestinal(GI) or lung origin with unresectable, locally advanced or metastatic disease[16].
| | Mode of action | The mammalian target of rapamycin(mTOR) pathway is one of the most clinically important molecular signalling networks to emerge over the past decade. It is the protein kinase at the core of this intricate and continually evolving pathway, controls cellular growth and behavior, impacting vital processes from immune reactivity to cancer progression[17, 18]. Everolimus is an mTOR inhibitor that binds with high affinity to the FK506 binding protein-12(FKBP-12), thereby forming a drug complex that inhibits the activation of mTOR[19-20]. This inhibition reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest and apoptosis. Everolimus also inhibits the expression of hypoxia-inducible factor, leading to a decrease in the expression of vascular endothelial growth factor[19, 20]. The result of everolimus inhibition of mTOR is a reduction in cell proliferation, angiogenesis, and glucose uptake. Since highly expression of mTOR is an important factor that promoting the cancer, blocking mTOR by Everolimus can effectively treat some kinds of cancer and the organ rejection due to immune response during/after organ transplantation[21, 22].
| | Pharmacokinetics | Oral everolimus is absorbed rapidly, and reaches peak concentration after 1.3–1.8 hours. Steady state is reached within 7 days, and steady-state peak and trough concentrations, and area under the concentration-time curve (AUC), are proportional to dosage. In adults, everolimus pharmacokinetic characteristics do not differ according to age, weight or sex, but bodyweight-adjusted dosages are necessary in children.
| | Adverse reactions | Some serious adverse reactions associated with Everolimus include non-infection pneumonitis, infections, severe hypersensitivity reactions, angioedema with concomitant use of ACE inhibitors, stomatitis, renal failure, impaired wound healing, metabolic disorders and myelosuppression[10]. Various common side effects include Bloating or swelling of the face, arms, hands, lower legs, or fee bloody nose, chest pain or tightness, chills, cough, decreased weight, diarrhea, difficult or labored breathing, difficulty with swallowing, fever, general feeling of discomfort or illness, hoarseness, lower back or side pain, painful or difficult urination, rapid weight gain, sores, ulcers, or white spots on the lips, tongue, or inside the mouth and tingling of the hands or feet[23]. Less common side effects include bleeding gums, bloody urine, blurred vision, burning, crawling, itching, numbness, prickling, or tingling feelings, coughing up blood, extreme tiredness or weakness, fast, pounding, or irregular heartbeat or pulse, increased thirst or urination, irregular breathing, loss of appetite, nausea, nervousness, nosebleeds, prolonged bleeding from cuts, red or black, tarry stools, red or dark brown urine, slow heartbeat, stomach ache, sweating, unusual tiredness or weakness and vomiting[23].
| | Warning and precautions | People who are allergic to Everolimus should be disabled. Since it can increase your risk of serious infections or getting certain cancers, such as lymphoma or skin cancer. The patients should ask their doctor about the specific risk[10, 23].
Patients who have the following conditions should consult for doctor for advice before administration: problem in digesting lactose or galactose(sugar); high cholesterol or triglycerides; liver disease; a heart transplantation; or skin cancer in them or their family members[23].
Since it may harm the unborn baby as well as affect fertility(the capability to have children), women should take effective birth control during administration of Everolimus and for at least eight weeks after stopping drugs. Should consult the doctor for advice if you want to or has become pregnant. It may also affect the fertility of men as well. It is generally not recommended to have breast-feed during the administration of Everolimus for women[10, 23].
| | References |
- https://www.drugbank.ca/drugs/DB01590
- https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm488028.htm
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022334s036lbl.pdf
- Junge, G., et al. "EVEROLIMUS, MTORC1 INHIBITION, AND IMPACT ON HEPATOCELLULAR CARCINOMA RECURRENCE AFTER LIVER TRANSPLANTATION-12, 24, AND 36 MONTHS DATA FROM 719 LTX RECIPIENTS." Transplant International 27(2014]: 23-23.
- Yao, J. C., et al. "Everolimus for advanced pancreatic neuroendocrine tumors. " New England Journal of Medicine 364.6(2011]: 514-23.
- Motzer, Robert J, et al. "Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma." Lancet Oncology 17.7(2016]: 917-927.
- Nachtnebel, A. "Everolimus[Afinitor] for advanced/metastatic kidney cancer."[2009].
- https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm254350.htm
- https://www.reuters.com/article/2012/07/20/novartis-afinitor-idUSL2E8IKD8B20120720
- https://www.rxlist.com/afinitor-drug.htm#indications_dosage
- Matin, Surena F. "Everolimus for the treatment of TSC-associated tumors." Community Oncology 9.12(2012]:361–362.
- Cappellano, A. M., et al. "Successful everolimus therapy for SEGA in pediatric patients with tuberous sclerosis complex." Childs Nervous System Chns Official Journal of the International Society for Pediatric Neurosurgery 29.12(2013]:2301-2305.
- Bilbao, Itxarone, et al. "Multiple indications for everolimus after liver transplantation in current clinical practice." World Journal of Transplantation 4.2(2014]: 122-132.
- Ganschow, Rainer, et al. "The role of everolimus in liver transplantation." Clinical & Experimental Gastroenterology 7.default(2014]:329-343.
- Dunn, C, and K. F. Croom. "Everolimus: a review of its use in renal and cardiac transplantation. " Drugs 66.4(2006]:547-570.
- https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm488028.htm
- Geissler, Edward K., H. J. Schlitt, and G. Thomas. "mTOR, Cancer and Transplantation." American Journal of Transplantation 8.11(2010]:2212-2218.
- Young, D. A., and C. L. Nickersonnutter. "mTOR--beyond transplantation. " Current Opinion in Pharmacology 5.4(2005]: 418-423.
- Anandappa, G., A. Hollingdale, and T. Eisen. "Everolimus – a new approach in the treatment of renal cell carcinoma." Cancer Management & Research 2.1(2010]:61.
- Raimondo, L., et al. "Everolimus induces Met inactivation by disrupting the FKBP12/Met complex:" Oncotarget 7.26(2016]:40073-40084.
- Sabatini, David M. "mTOR and cancer: insights into a complex relationship." Nature Reviews Cancer 6.9(2006]:729.
- Efeyan, Alejo, and D. M. Sabatini. "mTOR and cancer: many loops in one pathway." Current Opinion in Cell Biology 22.2(2010]:169-176.
- https://www.drugs.com/mtm/everolimus.html
| | Description | Everolimus, an oral immunosuppressant for the treatment of kidney and heart transplant rejection, is the 40-O-(2-hydroxyethyl) derivative of rapamycin. It has immunosuppressive properties similar to those of rapamycin, but with improved pharmacokinetic profile. Everolimus, like rapamycin, is a proliferation signal inhibitor that exerts its immunosuppressive effect by inhibiting the activation of p70 S6 kinase, thereby blocking growth factor-driven proliferation of T cells, B cells and vascular smooth muscle cells, and arresting cell cycle at the G1 phase. Inhibition of p70 S6 kinase activation by everolimus and rapamycin is mediated by their binding to FKBP12 (FK506 binding-protein 12). Everolimus inhibits FK506 binding to FKBP12 with an IC50 of 1.8–2.6 nM, and it is about 3- to 5-fold less potent than rapamycin (IC50=0.4–0.9 nM). The in vitro immunosuppressive activity of everolimus is also slightly less than that of rapamycin as demonstrated in a mixed lymphocyte reaction (MLR) assay (IC50=0.2–1.6 nM versus 0.07–0.5 nM, respectively) and in antigen-specific human helper T-cell clones (IC50=0.05–0.17nM versus 0.014–0.37nM, respectively). However, the in vivo immunosuppressive activity of oral everolimus 1–5 mg/ kg/day is similar to that of rapamycin at equivalent doses in rat models of renal or cardiac transplantation, localized graft-versus-host disease, and autoimmune glomerulonephritis. The recommended dosage of everolimus is 0.75 mg twice daily, and it is used in combination with cyclosporine microemulsion and corticosteroids. | | Chemical Properties | Off White Solid | | Originator | Novartis (Switzerland) | | Uses | Macrolide immunosuppressant; derivative of Rapamycin. Inhibits cytokine-mediated lymphocyte proliferation | | Uses | Everolimus is a semi-synthetic macrocyclic lactone prepared from rapamycin by selective alkylation of the 42-hydroxy group with a silyl-protected hydroxyethyl triflate moiety, followed by addition of an ethylhydroxy moiety to provide greater stability and bioavailability. Like all tacrolimus analogues, everolimus binds to receptor protein, FKBP12. The complex then binds to mTOR preventing it from interacting with target proteins. Everolimus is extensively cited in the literature with over 2,000 citations. | | Uses | Everolimus (IX) (SDZ-RAD), was developed by
Novartis as an immunosuppressant to be used in
conjunction with cyclosporin in transplantation allograft
rejection and was recently approved in the US in 2003.
Another natural product that had been approved for use in
transplantation is rapamycin (sirolimus) as an inejectable
agent. In an attempt to develop an orally bioavailable immunosuppressant agent, many companies attempted
modification of rapamycin itself. | | Definition | ChEBI: Everolimus is a macrocyclic lactone that is rapamycin in which the hydroxy group attached to the cyclohexyl moiety has been converted into the corresponding 2-hydroxyethyl ether. It is an immunosuppressant and antineoplastic agent. It has a role as an antineoplastic agent, an immunosuppressive agent, a mTOR inhibitor, an anticoronaviral agent and a geroprotector. It is a primary alcohol, a secondary alcohol, an ether, a cyclic ketone, a cyclic acetal and a macrolide lactam. It is functionally related to a member of sirolimus. | | Brand name | Certican | | General Description | Everolimus, sold under trade names including Zortress?, Certican, and Afinitor?, is an immunosuppressant drug used to prevent rejection of organ transplants and to treat renal cell cancer and other tumors. This Certified Spiking Solution? is suitable as starting material for calibrators, controls, or linearity standards for therapeutic drug monitoring or clinical and diagnostic testing of everolimus in patient whole blood samples by LC-MS/MS. | | Biological Activity | The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that, as part of two distinct complexes (mTORC1 and mTORC2), plays pivotal roles in intracellular signaling. Everolimus is a hydroxyethyl ether rapamycin derivative that inhibits mTOR signaling through both mTORC1 and mTORC2 when added to cells at 20 nM. It is orally available and shows improved pharmacokinetics and pharmacodynamics over rapamycin. Through its inhibition of mTOR, everolimus inhibits cell proliferation, metabolism, and angiogenesis in certain types of cancer. It also acts as an immunosuppressive agent in the context of organ transplantation. | | Biochem/physiol Actions | Everolimus, the 40-O-(2-hydroxyethyl) derivative of rapamycin (sirolimus), is a potent and selective inhibitor of mechanistic target of rapamycin (mTOR). Everolimus is selective for the mTORC1 protein complex. Everolimus exhibit potent immunosuppressive and anticancer activities. | | Synthesis | Everolimus (IX) was
discovered by Sandoz (Novartis) scientists by modifying
rapamycin drug in the 40-hydroxyl position. Thus,
treatment of rapamycin (84) with t-butyldimethylsilyloxy
ethyl triflate in the presence of 2,6-lutidine at 60??C for 3.5
hrs gave ether 85. Deprotection of the silyl group was done
by treating silyloxy ether 85 in methanol with 2N HCl to
give the product IX (everolimus), which was purified by
chromatography. No yields were given for the reactions. 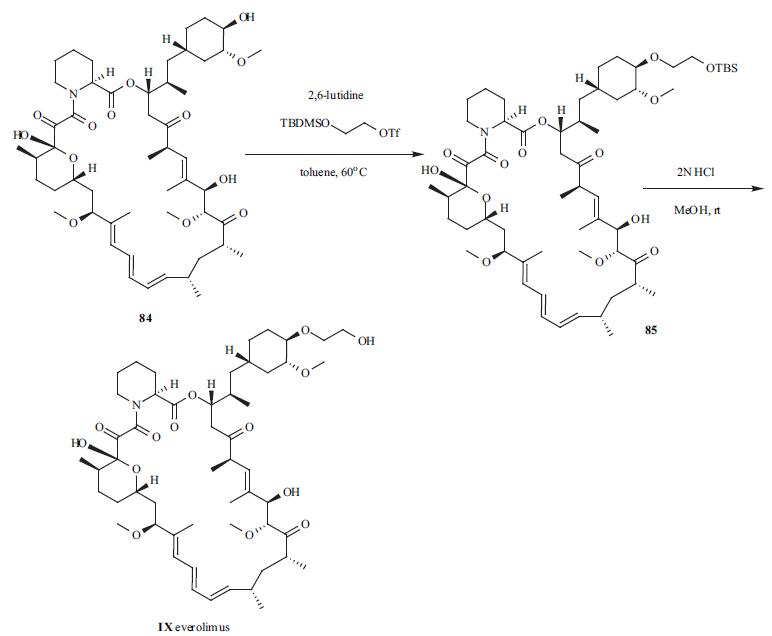
| | target | mTOR (FKBP12) | | Drug interactions | Potentially hazardous interactions with other drugs
ACE-Is: increased risk of angioedema.
Antibacterials: erythromycin, clarithromycin and
telithromycin increase everolimus levels - avoid
with clarithromycin and telithromycin; rifampicin
decreases everolimus levels by factor of 3.
Antidepressants: St John’s wort decreases everolimus
levels.
Antifungals: concentration increased by ketoconazole
and possibly itraconazole, posaconazole and
voriconazole - avoid.
Antipsychotics: increased risk of agranulocytosis
with clozapine - avoid.
Antivirals: concentration possibly increased by
atazanavir, darunavir, indinavir, ritonavir and
saquinavir - avoid; concentration significantly
increased by dasabuvir and ombitasvir/paritaprevir/
ritonavir - avoid concomitant use.
Calcium channel blockers: concentration of both
drugs increased with verapamil.
Ciclosporin: increases everolimus AUC by 168% and
Cmax by 82%.
Cytotoxics: concentration increased by imatinib -
consider reducing everolimus dose.
Grapefruit juice: increases everolimus levels. | | Metabolism | Everolimus is metabolised in the liver and to some
extent in the gastrointestinal wall, and is a substrate of
P-glycoprotein and the cytochrome P450 isoenzyme
CYP3A4. Six main metabolites of everolimus have
been detected in human blood, including three
monohydroxylated metabolites, two hydrolytic ring�opened products, and a phosphatidylcholine conjugate
of everolimus. These metabolites were shown to have
approximately 100 times less activity than everolimus
itself. Following the administration of a single dose of
radiolabelled everolimus, 80% of the radioactivity was recovered from the faeces, while 5% was excreted in the
urine. The parent substance was not detected in urine or
faeces. | | storage | Store at -20°C | | References | 1) Schuurman et al. (1997), SDZ RAD, a new rapamycin derivative: synergism with cyclosporine; Transplantation, 64 32
2) Neuhaus et al. (2001), mTOR inhibitors: an overview; Liver Transpl., 7 473
3) Kahan et al. (2002), Therapeutic drug monitoring of immunosuppressant drugs in clinical practice; Clin. Ther., 24 330 |
| | Everolimus Preparation Products And Raw materials |
|