Alginic acid - Application in Meat Manufacturing Industry
Mar 10,2022
Alginic acid is a copolymer of two uronic acids, mannuronic and guluronic, is extracted from brown seaweed, and has the unique property of forming a gel in the presence of a divalent cation ( Fig. 3.6 ). When producing alginates, the uronic acids are converted into their salt forms, mannuronate (M) and guluronate (G) through a neutralization step. In the natural state, M and G units can be linked together in one of three blocks: MM, GG, or MG (GM). The proportion, distribution, and length of these blocks determine the chemical and physical properties of the molecules. The reactivity with calcium and the consequent gelling capacity is a direct function of the average length of the G blocks. Alginates containing high levels of GG fractions possess the ability to form strong gels. These fractions are more abundant in the stems of the seaweed, while the leaves contain alginic acids with higher proportions of M fractions. Functional Characteristics and Properties Alginates are soluble in cold water and therefore do not require heating to form gels. These gels develop upon exposure to multivalent cations. Because of its commonality and safety, calcium is usually the cation of choice.
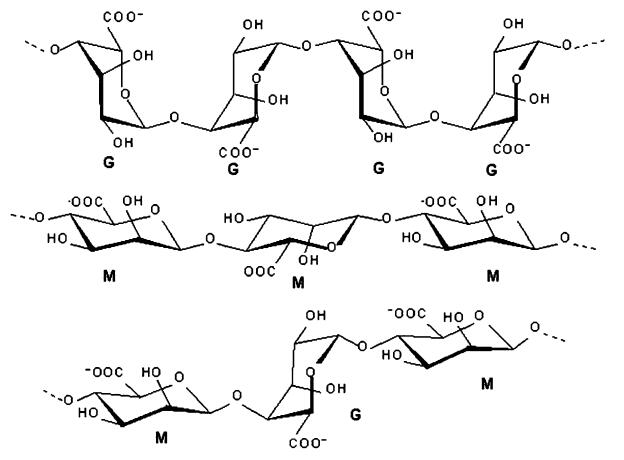
Fig. 3.6 Basic structures of G-block and M-block components of alginic acid (courtesy of FMC Corporation, Philadelphia, PA, USA).
The solubility of the calcium source is one of the criteria used to control the rate
of gel formation. A calcium salt solution will cause alginate in solution to gel on
contact. Continuous exposure of the alginate to the calcium solution will increase
the firmness of the gel, as more calcium diffuses into the gel and binds to the G
blocks within the alginate structure. This process, used for encapsulating liquids,
can be used for forming a gel layer, or skin, on the surface of meat products. The
addition of alginate to a meat blend followed by bathing or showering in a calcium
chloride solution results in the formation of skin on the surface. Extended exposure
time to the calcium chloride solution will increase the thickness of the skin and, if
long enough, will eventually create a gelled structure throughout the product due to
calcium diffusivity. Time of complete gelling will be dependent on the thickness of
the structure. This process of forming an alginate gel is referred to as external
gelling.
For internal gelling, sodium alginate and the calcium source are added directly to the meat mixture and allowed to form a gel over time. The success of this process is based on ensuring the complete hydration of alginate and the proper timing of calcium release, which can be controlled by the solubility of the calcium source or by the addition of a sequestrant. A gel formed too quickly has the potential to break apart during the mixing or stuffing process. Because calcium alginate gels do not re-heal, if the gel is broken during the structuring process, it could appear that it did not form in the first place. Calcium chloride would not be the calcium source of choice for this type of application. More commonly, a less soluble calcium source is used.
The process for making structured meat using alginate gels was patented by
researchers at Colorado State University (Schmidt & Means, 1986) . The patent
describes a method to form structured meat products using sodium alginate, calcium
carbonate, and glucono-delta-lactone (GdL). Sodium alginate, once added to
the meat block, is hydrated by the moisture in the meat. Calcium carbonate has low
solubility and gives the processor time to stuff the meat mixture into casings. GdL
decreases pH over time, which causes the solubility of calcium carbonate to
increase, thus releasing calcium and resulting in the formation of an alginate gel
able to entrap the meat particles. Once the gel has set, the product is sliced into
steaks or chops. Since the calcium alginate gel is heat stable, the product will not
fall apart during cooking.
To assure optimum gelling properties, it is essential that the alginate be fully
hydrated and evenly distributed in the mixture. Adding alginate directly to the meat
source allows the alginate to hydrate by drawing moisture from the meat. To speed
up gel formation, sodium alginate should be hydrated with water prior to addition
to the meat mixture.
The action of a sequestrant is to chelate calcium, with the objective being to slow
down the gelation mechanism of sodium alginate. There is evidence that slowing
gelation can also increase gel strength, most likely due to a more ordered formation
of the gel structure. Conditions that retard intermolecular interactions will result in
a more homogeneous and regular network and consequently a stronger gel (Bernal
et al., 1987) . The sequestrant most often used is phosphate. The effectiveness of the sequestrant is influenced by solubility. For example, at equal phosphate levels,
sodium tripolyphosphate is more effective than tetrasodium pyrophosphate at
retarding gel formation.
The texture of structured meat is a primary focus among meat processors.
Structured meat should be superior to ground meat, without having a gel-like
texture. The mechanical properties of structured meat are influenced by size and
shape of the raw material (Berry & Civille, 1986) as well as sodium alginate/
calcium ratio (Trout, Chen, & Dale, 1990) . Devatkal and Mendiratta (2001) found
the mechanical properties of structured pork rolls were superior for salt-phosphate
rolls in the cooked state but were better for alginate/lactate gels in the raw
state.
Means and Schmidt (1986) indicated the ideal alginate:calcium ratio was 2.5 g alginate:0.18 g calcium ion. Ensor, Ernst, Sofos, and Schmidt (1986) made acceptable structured turkey meat using 0.4–1.0% sodium alginate, 0.075–0.1875% calcium carbonate, and 0.6% lactate. Later, Ensor, Sofos, and Schmidt (1990) found 0.4% sodium alginate, 0.075% calcium carbonate, and 0.6% lactate were optimal for use in structured meat products. Basic differences in optimal use level will result with different raw materials. As the divalent ion concentration increases, there is an increase in the strength of the alginate gels, resulting from multiple molecular interactions. Along with the increase in gel strength, however, increases in calcium level can also result in an increase in syneresis or purge, as stronger gels will tend to push more water out of the structure. Decreasing the amount of calcium added to the formula will lower the gel strength and reduce the associated syneresis. The gelling mechanism for alginate is well known. However, the extent to which the gelling mechanism is influenced by the presence of muscle proteins has not been elucidated. A few studies have been conducted to determine if proteins are part of the gelling mechanism in meat applications. Imeson, Ledward, and Mitchell (1977) observed considerable changes in UV–visible absorption of myoglobin and serum albumin caused by alginate and certain other polysaccharides. These absorption spectra indicated some type of interaction between alginates and proteins, which the authors attributed to electrostatic forces. Bernal et al. (1987) later confirmed the involvement of electrostatic forces with crude myofibrillar protein. However, Xiong and Blanchard (1993) showed no change in absorption spectra when scanning various combinations of salt-soluble proteins (SSP) and alginate but did find a reduction in the gelling suspension. Offering an explanation for this unpredicted result, the authors surmised that there may be minimal hydrocolloid– protein interactions in the initial phase of SSP gelation (structural unfolding) but, as the gelling point is approached, alginate interferes with the gel network formation. It could also be that alginate has no influence on hydrophobic groups within the protein molecule but has some influence on electrostatic bonding. This influence of alginate could also be observed in the final gelled product as lower gel firmness.
Meat products using alginate gel technology should be treated like ground meat from a food safety standpoint (Ortega-Valenzuela, Phebus, & Thippareddi, 2001) and, therefore, should be cooked to internal temperatures that ensure product safety. This could limit their acceptance as replacements for intact muscle. Making products using batch-type systems is another limitation for the acceptance of alginate gel technology. There are no known methods to process structured meat products on a continuous basis. Wotherspoon (1988) was granted a patent teaching the continuous production of reconstituted pet food chunks from comminuted materials. Modifications of this procedure may be used to make raw materials for further processed meat products.
Besides increasing the value of under-utilized muscles, meat processors can take advantage of alginate characteristics in other ways. Mechanically separated muscle has limited use due to its contribution to a reduction in firmness of the final product. Using alginates to structure these products improves the texture of the final product, with the added advantage of improving cook yield (Lamkey, 2006) . Sodium alginate can be used to structure mixtures of protein and water, where the protein source can range from 25% to 70% of the formulation. The boundaries are limited only by the texture desired. Reducing the amount of protein creates a firm, gel-like texture, while increasing the protein results in softer but more meat-like texture.
Lin and Keeton (1998) suggested a combination of sodium alginate and carrageenan can replace fat in precooked beef patties with similar textural properties. In this case, alginate is used in its nongelled form. Means and Schmidt (1986) , however, found off-flavors associated with higher alginate levels and attributed it to nongelled alginate. It is, therefore, recommended that sensory attributes on nongelled alginate products be evaluated.
Alginate, like most carbohydrates, can also form films used to protect the surface of fresh meat products. A patent by Earle and McKee (1976) describes the use of alginate in combination with starch to form a coating for the protection of fresh beef carcasses. Williams, Oblinger, and West (1978) concluded that a calcium alginate coating significantly decreased shrink in coated vs. uncoated steaks and also helped maintain the oxymyoglobin color for a longer period of time. Warmed-over flavor was eliminated in precooked, alginate-coated patties as judged by sensory scores and TBA values (Wanstedt, Siedeman, Donnelly, & Quenzer, 1981) . Patties having a calcium alginate coating were juicier and more desirable in texture and overall palatability than raw or precooked patties without calcium alginate. A reduction in oxidation as well as an improvement in moisture retention were shown to be advantages of having alginate in a presoak for rabbit muscle that was irradiated with 5 KGy.
Regulatory Status
Based on current USDA regulations, a mixture of sodium alginate (not to exceed 1% of product formulation), calcium carbonate (not to exceed 0.2%), and calcium lactate/lactic acid (or GdL) (not to exceed 0.3%) is permitted for use in restructured meat food products to bind meat pieces. The entire mixture is not to exceed 1.5% of product at formulation and it must be added dry (CFR, 2007b) . For ground and formed raw or cooked poultry pieces, sodium alginate cannot exceed 0.8%, calcium carbonate cannot exceed 0.15%, and calcium lactate/lactic acid cannot exceed 0.6%. The entire mixture cannot exceed 1.55% of product formulation and it also must be added dry (CFR, 2007b) .
The USDA also allows for the application of an alginate film on freshly dressed meat carcasses to reduce cooler shrinkage and help protect surface (CFR, 2007b) . A mixture containing water, sodium alginate, calcium chloride, carboxymethylcellulose, and corn syrup solids may not exceed 1.5% of hot carcass weight when applied and chilled weight cannot exceed hot carcass weight (no added water). In the European Union, sodium alginate is listed in the group of approved emulsifiers, thickeners, stabilizers, and gelling agents for use “quantum satis” and has been assigned the reference number E 401 (European Parliament and Council, 2006) .
- Related articles
- Related Qustion
Increasing the value of under-utilized muscles of food animals has been the target of many published reports involving alginate. Many of these muscles, usually originating from the hind leg or shoulder area.....
Mar 10,2022Organic ChemistryKonjac is the generic name for the flour formed from grinding the root of the Amorphophallus konjac (elephant yam) plant (FMC Biopolymer, 1994) . It is a food ingredient containing a high-molecular weight polysaccharide.....
Mar 10,2022Food AdditivesAlginic acid
9005-32-7You may like
- The new research on Silychristin
Apr 29, 2024
- What is chlorella spermidine?
Apr 19, 2024
- Does icariin help with pain?
Apr 18, 2024
- Alginic Acid
-

- $0.00 / 1Kg
- 2024-03-21
- CAS:9005-32-7
- Min. Order: 1Kg
- Purity: 99.9%
- Supply Ability: 200tons
- Alginic acid
-

- $30.00 / 1kg
- 2023-09-13
- CAS:9005-32-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons
- Alginic acid
-

- $30.00 / 1KG
- 2023-09-06
- CAS:9005-32-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg




