Baricitinib: Pharmacodynamics and pharmacokinetics
Jun 7,2023
General description
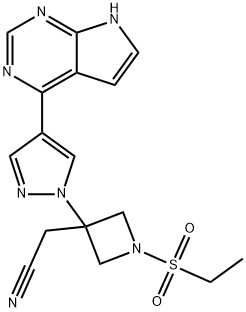
Baricitinib is an oral, targeted synthetic DMARD that inhibits JAK1 and JAK2, which are implicated in the pathogenesis of rheumatoid arthritis (RA). This novel, small molecule is approved for use as monotherapy, or in combination with methotrexate, for the treatment of adults with moderate to severe active RA who responded inadequately to or were intolerant of C1 DMARD. baricitinib treatment also slowed structural joint damage in DMARD- naive patients and in those with an inadequate response to methotrexate and csDMARDs. Baricitinib plus methotrexate was more effective than adalimumab plus methotrexate in patients with an inadequate response to methotrexate. The onset of these benefits was generally rapid and sustained over time. Baricitinib was generally well tolerated during up to 5.5 years’ treatment; the most commonly reported adverse drug reactions were upper respiratory tract infections, increased LDL cholesterol, nausea and thrombocytosis. Thus, once-daily baricitinib, as monotherapy or in combination with methotrexate, is an effective and generally well tolerated emerging treatment for patients with moderate to severe active RA who have responded inadequately to or are intolerant of C 1 DMARD, and extends the options available for this population. Its appearance is as follows:

Figure 1 Appearance of Baricitinib
Pharmacodynamics
Baricitinib is an ATP competitive kinase inhibitor that selectively, potently and reversibly inhibits JAK1 and JAK2, with IC50 values of 5.9 and 5.7 nmol/L [1]. Baricitinib also inhibited the effects of JAK3, TyK2, c-MET and Chk2 kinases (respective IC50 values of ≈ 560, 53, >10,000, >1000 nmol/L) [1]. The role of JAKs in the pathogenesis of RA has been established, as they transduce intracellular signals for various cytokines and growth factors involved in inflammation, haematopoiesis, and immune function [2]. In the signaling pathway, signal transducers and activators of transcription (STATs) are phosphorylated and activated by JAKs, thereby activating gene expression within the cell [2]. Baricitinib modulates these intracellular signaling pathways, reducing the phosphorylation and activation of STATs by partially inhibiting JAK1 and JAK2. Baricitinib demonstrated dose-dependent efficacy in patients with moderate to severe RA for up to 24 weeks [3]. Results of population pharmacokinetic/pharmacodynamic models demonstrated that the optimum benefit-risk balance was offered by the 4 mg once daily dosage; an acceptable benefit-risk profile was also evident with the lower dosage of baricitinib (i.e. 2 mg once daily) [3].
Pharmacokinetics
Oral baricitinib exhibits dose-linear and time-independent pharmacokinetics [1]. Following oral administration, the maximum plasma concentration (Cmax) of baricitinib is reached in a median time of ≈ 1 h and the absolute bioavailability is 79%, with food having no clinically relevant effects on its pharmacokinetics [4]. In patients with RA, steady-state Cmax and area under the concentration-time curve (AUC) values were 1.4- and 2-fold higher than in healthy volunteers. Baricitinib is ≈ 50% bound to plasma proteins, and has a mean volume of distribution of ≈ 76 L following an intravenous infusion [4]. Metabolism of baricitinib is mediated by CYP3A4, but with 10% of the administered dose undergoing biotransformation [4]. Baricitinib is predominantly eliminated via the renal route, with ≈ 75 and 20% of the administered dose eliminated in the urine and faeces. In patients with RA, the mean apparent clearance was 9.42 L/h and the half-life was 12.5 h. In patients with mild or moderate renal impairment, baricitinib exposure is increased compared with patients with normal function [4]. A reduced dosage of baricitinib 2 mg once daily is recommended in patients with creatinine clearance (CLCR) of 30–60 mL/min (moderate renal impairment) and the use of baricitinib is not recommended in patients with CLCR of 30 mL/min (severe renal impairment) [4].
Baricitinib is a substrate for CYP3A4, OAT3, P-gp, BCRP and MATE2-K, in vitro [5]. Following co-administration of baricitinib with probenecid (a strong OAT3 inhibitor), a twofold increase in AUC of baricitinib was evident [5]; a dosage reduction to 2 mg once daily is recommended in patients concomitantly receiving strong OAT3 inhibitors. Given the potential increase in baricitinib exposure, caution is recommended when leflunomide (prodrug) or its active form, teriflunomide (a weak OAT3 inhibitor), are co-administered with baricitinib.
References
[1]Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184(9):5298–307.
[2]European Medicines Agency. Olumiant (baricitinib): EU assess- ment report. 2016. https://www.ema.europa.eu. Accessed 10 Apr 2018.
[3]Zhang X, Chua L, Ernest C 2nd, et al. Dose/exposure-response modeling to support dosing recommendation for phase 3 devel- opment of baricitinib in patients with rheumatoid arthritis. CPT Pharmacomet Syst Pharmacol. 2017;6(12):804–13.
[4]European Medicines Agency. Olumiant (baricitinib) film-coated tablets: EU summary of product characteristics. 2017. https:// www.ema.europa.eu/. Accessed 10 Apr 2018.
[5]Posada MM, Cannady EA, Payne CD, et al. Prediction of trans- porter-mediated drug–drug interactions for baricitinib. Clin Transl Sci. 2017;10(6):509–19.
- Related articles
- Related Qustion
- The pharmacologic action of Baricitinib Sep 17, 2019
Baricitinib is a selective and reversible Janus kinase 1 (JAK1) and 2 (JAK2) inhibitor. Janus kinases belong to the tyrosine protein kinase family and play an important role in the proinflammatory pathway signalling that is frequently over-
Docetaxel is a chemotherapy drug that has potent anti-cancer activity against various tumors and has promising effects in promoting the biogenesis of high-density lipoprotein to prevent heart disease.....
Jun 7,2023APICarbamazepine is a widely used antiepileptic drug that offers clinicians many choices for treating epilepsy and trigeminal neuralgia. However, carbamazepine can cause several adverse drug reactions.....
Jun 7,2023APIBaricitinib
1187594-09-7You may like
- Baricitinib
-
- $2.00 / 1kg
- 2024-04-29
- CAS:1187594-09-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1200KG/M
- Baricitinib
-

- $80.00 / 1KG
- 2024-04-28
- CAS:1187594-09-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500
- Baricitinib
-

- $100.00 / 1KG
- 2024-04-28
- CAS:1187594-09-7
- Min. Order: 1KG
- Purity: 99.9%
- Supply Ability: 1000