Benzylamine: Properties, Preparation and Applications
May 24,2023
General Description
Benzylamine is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth.

Figure 1. Properties of Benzylamine
Chemical Properties
Benzylamine is a stronger base than its toluidine isomers and reacts strongly alkaline even if diluted with some water (pH 11.6 in water at 100 g/L). It forms addition compounds with, e.g., phenol (1 : 3, mp 15.3 oC), p-cresol (1 : 1, mp 6 oC), and formic acid (1 : 1, mp 81 oC). Examples of the salts of benzylamine are the hydrochloride, C6H5CH2NH3+Cl-, mp 260 oC (decomp.), and the picrate, mp 194 oC. Benzylamine absorbs carbon dioxide from the air to form the solid carbamic acid salt. If benzylamine is boiled with glacial acetic acid, N-acetylbenzylamine is formed. Benzylamine reacts with isocyanates to give substituted ureas, C6H5CH2NHCONHR. Benzylamine is nitrated on the aromatic nucleus (8 % ortho-, 49 % meta-, 43 % para-nitrobenzylamine) by nitric acid. Catalytic hydrogenation gives hexahydrobenzylamine, C6H11CH2NH2.1
Applications and Reactions
Benzylamine is used in synthetic textiles, in paints, and as corrosion inhibitor. It is also used as an intermediate in the production of pharmaceutical active substances and compounds for plant and material protection. In this cases benzylamine is often used as "protected nitrogen". Benzylamine Hydrochloride is medicine used as an anesthetic or dilator. In respiratory disorders, this substance acts as a bronchodilator. In dermatologic medicines, this substance acts as an anti-pruritic and anti-psoriatic. It is also used in mouthwash, sore throat treatments and motion sickness medicines.2-4
Preparation
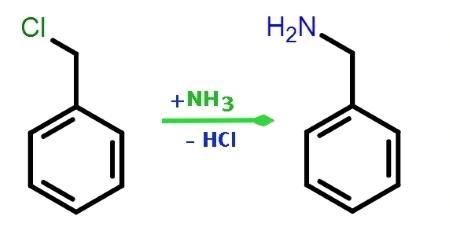
Figure 2. Preparation of Benzylamine
Benzylamine is produced by the reaction of benzyl chloride with ammonia in aqueous solution. The catalytic hydrogenation of benzonitrile and the reaction of benzaldehyde with ammonia in the presence of hydrogen and catalysts are alternatives. Both processes use some organic solvent in the reaction mixture. In the benzaldehyde process, 8 kg of Raney nickel that has been kept under water (corresponding to about 4 kg of 100 % Ni), 10 g of glacial acetic acid, and 110 kg of ammonia are added to a mixture of 250 kg of methanol and 500 kg of benzaldehyde. The resulting batch is hydrogenated in a 1200-L steel autoclave at 100 oC and 15 MPa. The process takes 3 – 5 h. The hydrogen consumed in the reaction is made up at intervals of about 10 min. The contents of the reaction vessel are maintained at the same temperature and pressure for 30 min after hydrogen consumption ends. The catalyst is then separated from the reaction products on a pressure filter. The filtrate contains 470 kg of benzylamine, about 1 kg of benzyl alcohol, less than 1 kg of dibenzylamine, and about 2 kg of Schiff base (N-benzalbenzylamine), in addition to methanol, reaction water, and ammonia. The yield is 93 %. Vacuum distillation yields benzylamine of the required technical purity. Benzylamine has been isolated as a natural compound in the leaves and flowers of Reseda media.5
Toxicology
Benzylamine is readily biodegradable. It is harmful in aquatic environment [German class for hazards in aquatic environment: 1 (weak effect) (WGK 1)]. Acute toxicity: Pseudomonas fluorescens (bacteria) 500 mg (EC0); Scenedesmus quadricauda (algae) 6 mg (96-h EC10); Daphnia magna 60 mg (48-h EC50); Leuciscus idus (fish) 20 mg (48-h EC0); Pimephals promelas (fish) 102 mg (96-h EC50). Benzylamine causes burns to skin and eyes and may cause sensitization. Rinse immediately with plenty of water. Wash skin also with soap. When used regularly a 5 % aqueous solution of acetic acid should be available for neutralization of contaminated skin. If swallowed dilute by drinking water, rinse mouth. If risk of inhalation is given, use ABEK filters in breathing masks (DIN 3181). Benzylamine is Ames test negative (no mutagenic effect). Acute oral toxicity: LD50 1130 mg/ kg (rat). Acute percutaneous toxicity: LD501340 mg/kg (rat). Toxicity class 3 (Switzerland). All rats inhaling benzylamine for 3 h survived a two-week period (full body exposure). After full body exposure for 5 h, 17 % died.6
References
1. Merck, Data Sheet for Benzylamine for Synthesis, 801812, Catalogue 1999.
2. B. Wanderott, Z. Metallkd. 56 (1965) no. 1, 63 – 64.
3. H. Ippen: Index Pharmacorum, Thieme Verlag, Stuttgart 1968.
4. T. W. Green (Ed.): Protective Groups in Organic Synthesis, Wiley, New York, 1981, p. 272.
5. A. T. Mason, J. Chem. Soc. 63 (1893) 1311. California Research Corp., US 2987548, 1961.
6. Lanxess Safety Data Sheet Benzylamine 012193 (Sept. 26, 2005).
- Related articles
- Related Qustion
- Uses of Benzylamine Jan 4, 2022
Benzylamine is belongs to the class of organic compounds known as phenylmethylamines. These are compounds containing a phenylmethtylamine moiety, which consists of a phenyl group substituted by an methanamine.This colorless water-soluble li
Urolithin A (UA) is produced by gut microflora from foods rich in ellagitannins. UA has been shown to improve mitochondrial health preclinically and in humans. Not everyone has a microbiome capable of producing UA, making supplementation wi....
May 23,2023Biochemical EngineeringPotassium Formate is a useful chemical compound in the production of potassium metal and in the oil and gas industry, often in aqueous solution.....
May 24,2023Inorganic saltsBenzylamine
100-46-9You may like
- Benzylamine
-

- $10.00 / 1KG
- 2024-01-08
- CAS:100-46-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000tons
- Benzylamine
-
- $50.00 / 1KG
- 2023-12-23
- CAS:100-46-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Benzylamine
-

- $0.00 / 200kgs
- 2023-09-23
- CAS:100-46-9
- Min. Order: 1000kgs
- Purity: 99%
- Supply Ability: 20 tons





