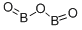Boron oxide:Uses,Production,Reactions
Feb 28,2023
Boron oxide is a colorless transparent solid, almost always glassy (amorphous), which can be crystallized only with great difficulty. It is also called boric oxide or boria.It has many important industrial applications, chiefly in ceramics as a flux for glazes and enamels and in the production of glasses.

Production
Boron oxide is produced by treating borax with sulfuric acid in a fusion furnace. At temperatures above 750℃, the molten boron oxide layer separates out from sodium sulfate. It is then decanted, cooled and obtained in 96%-97% purity. Another method is heating boric acid above B300℃.
Boric acid will initially decompose into steam (H2O(g)) and metaboric acid (HBO2) at around 170℃, and further heating above 300℃ will produce more steam and diboron trioxide. The reactions are:
H3BO3→HBO2+H2O and 2HBO2→B2O3 + H2O
Boron monoxide (B2O) is a chemical compound of boron and oxygen. Two experimental studies have proposed existence of diamond-like and graphite-like B2O, as for boron nitride and carbon solids. However, a later, systematic, experimental study of boron oxide phase diagram suggests that B2O is unstable.
Reactions
Molten boron oxide attacks silicates. Containers can be passivated internally with a graphitized carbon layer obtained by thermal decomposition of acetylene.
Uses
Major component of borosilicate glass;
Fluxing agent for glass and enamels;
Starting material for synthesizing other boron compounds such as boron carbide;
An additive used in glass fibres (optical fibres);
The inert capping layer in the Liquid Encapsulation Czochralski process for the production of gallium arsenide single crystal;
As an acid catalyst in organic synthesis.
- Related articles
- Related Qustion
Glucose oxidase is an enzyme found in molds such as Penicillium notatum and honey. It can catalyze D-glucose and O2 to produce D-gluconic acid (δ- Lactone) and H2O2.....
Feb 28,2023APICarbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual a....
Feb 28,2023Inorganic chemistryBoron oxide
1303-86-2You may like
- Crystal Structure of Aluminum Selenide
Apr 23, 2024
- Crystal Structure and Property of Tin Selenide
Apr 23, 2024
- Crystal Structure of Uranium Carbide
Apr 22, 2024
- Boron oxide
-

- $110.00 / 10kilograms
- 2024-04-30
- CAS:1303-86-2
- Min. Order: 10kilograms
- Purity: 99%
- Supply Ability: 10
- Boron oxide
-

- $20.00 / 1kg
- 2023-07-12
- CAS:1303-86-2
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 50000tons
- Boron oxide
-

- $20.00 / 1kg
- 2023-06-30
- CAS:1303-86-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000tons