Calicheamicin and Calicheamicin gamma 1I
Apr 15,2024
Introduction
Calicheamicins are bioactive molecules produced by bacteria of the Micromonospora species and constitute a class of powerful enedynes (that is, organic compounds containing two triple bonds and one double bond) endowed with antibiotic effect against both Gram+ and Gram− bacteria and anticancer activity. It is a highly potent antitumor antibiotic initially isolated from the actinomycete Micromonospora echinospora. It binds to the minor groove of DNA and cleaves double-stranded.
Calicheamicin is the active payload in two approved ADCs:
Besponsa, based on the humanized IgG4 Ab Inotuzumab targeting the B cell receptor CD22 and employed for the treatment of relapsed/refractory B cell acute lymphoblastic leukemia, and Mylotarg, based on the humanized IgG4 Ab Gemtuzuma targeting the myeloid cell surface antigen CD33 and used to treat relapsed/refractory acute myeloid leukemia[1].
Mode of action
Once conjugated to an Ab, calicheamicin release relies on a two-step process: first, the hydrazone is selectively released in the acidic intracellular environment, then the disulfide link is reduced by intracellular glutathione; this, in turn, activates an intramolecular 1,4-addition reaction followed by a Bergman cyclization, leading to the formation of radical species. This reactive intermediate binds within the DNA double helix minor groove, ultimately producing DNA double-strand breaks and, hence, inducing cell death.
Calicheamicin gamma 1I
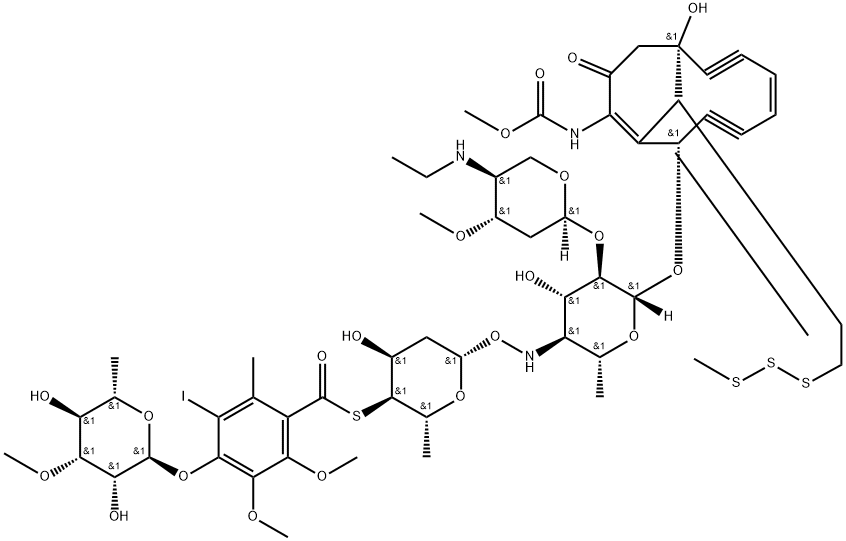
Calicheamicin gamma 1I is a discovered diyne-ene--containing antitumor antibiotic with considerable potency against murine tumors.
It has been isolated from fermentation media to which sodium iodide has been added. Its chemical structure consists of a core bicyclo (7.3.1) tridecadiynene moiety (ring R) attached to an aryl tetrasaccharide chain. This chain consists of linked elements involving a hydroxylamino sugar (ring A), a thio sugar (ring B), a thiobenzoate (ring C), and a rhamnose sugar (ring D). An ethylamino sugar (ring E) is connected to sugar A through a glycosidic linkage. The enediyne core and attached trisaccharide segments are common to esperamicin A1 (R, A, B, and C rings) and calicheamicin g1 I (R, A, B, and E rings), including the novel NH-O connectivity linking the A and B sugars [2].
In vitro, this drug interacts with double-helical DNA in the minor groove and causes site-specific double-stranded cleavage. It is proposed that the observed cleavage specificity is a result of a unique fit of the drug and DNA followed by the generation of a nondiffusible 1,4-dehydrobenzene--diradical species that initiates oxidative strand scission by hydrogen abstraction on the deoxyribose ring. The ability of calicheamicin gamma 1I to cause double-strand cuts at very low concentrations may account for its potent antitumor activity[3].
References
[1] Cannon, J. “Site-Specific Drug Delivery Utilizing Monoclonal Antibodies.”Advanced and Modern Approaches for Drug Delivery (2023): 649-681.
[2] R A Kumar, D J Patel, N Ikemoto. “Solution structure of the calicheamicin gamma 1I-DNA complex.” Journal of Molecular Biology 265 2 (1997): 187–201.
[3] Nada Zein. “Calicheamicin γ1I: an Antitumor Antibiotic That Cleaves Double-Stranded DNA Site Specifically.” Science 240 4856 (1988).
- Related articles
- Related Qustion
- Calicheamicin gamma1 Unveiled: Pathway from Microbial Marvel to Therapeutic Powerhouse Apr 23, 2024
Calicheamicin gamma1, a marvel of natural product chemistry, has emerged as a beacon of hope in the ongoing battle against cancer.
- Calicheamicin: Bioactivity, Mechanism of action, Application Dec 13, 2022
Calicheamicin is a potent cytotoxic agent that can cause DNA double strand breaks.
rifluoroacetic acid (TFA) is widely used as a solvent, catalyst and reagent in organic synthesis.....
Apr 15,2024APIChromium borides have been studied extensively both experimentally and theoretically. This article will show the crystal structure of CrB system.....
Apr 15,2024Inorganic chemistryCalicheamicin
108212-75-5You may like
- Calicheamicin gamma1
-
- $8.00 / 1kg
- 2024-04-05
- CAS:108212-75-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- calicheamicin gamma(1)I
-

- $7.00 / 1KG
- 2020-02-12
- CAS: 108212-75-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG