EDTA : a chelating agent and anticoagulant
Apr 28,2024
Why is EDTA used as a chelating agent?
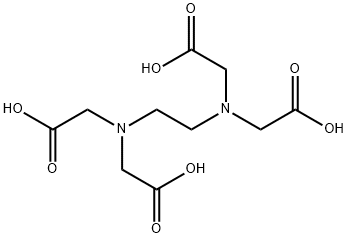
Ethylenediaminetetraacetic acid (EDTA) refers to the chelating agent with the formula (HO2CCH2)2 NCH2CH2N (CH2CO2H)2. This amino acid is widely used to sequester di- and trivalent metal ions. EDTA binds to metals through four carboxylate and two amine groups. EDTA forms especially strong complexes with Mn (II), Cu (II), Fe (III), and Co (III). It is mostly synthesized from 1, 2-diaminoethane (ethylene diamine), formaldehyde, water, and sodium cyanide. This yields the tetrasodium salt, which can be converted into the acidic forms by acidification. It is a polyamino carboxylic acid and a colorless, water-soluble solid widely used to dissolve lime scale[1].
It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand and chelating agent, i.e., its ability to sequester metal ions such as Ca2+ and Fe3+. After being bound by EDTA, metal ions remain in the solution but exhibit diminished reactivity. EDTA is produced as several salts, notably disodium EDTA and calcium disodium EDTA. EDTA reacts with the calcium ions in dentine and forms soluble calcium chelates. It has been reported that EDTA decalcified dentin to a depth of 20-30 μm in 5 min.
How does EDTA anticoagulant?
Anticoagulants are used to prevent clot formation both in vitro and in vivo. In the specific field of in vitro diagnostics, anticoagulants are commonly added to collection tubes to maintain blood in the fluid state for hematological testing or to obtain suitable plasma for coagulation and clinical chemistry analyses. EDTA is a polyprotic acid containing four carboxylic acid groups and two amine groups with lone-pair electrons that chelate calcium and several other metal ions[2]. Calcium is necessary for a wide range of enzyme reactions of the coagulation cascade, and its removal irreversibly prevents blood from clotting within the collection tube. It inhibits clotting by removing or chelating calcium from the blood. EDTA's most crucial advantage is that it does not distort blood cells, making it ideal for most hematological tests. It is known to cause erroneous results of platelet counts by automated hematological analyzers yielding low platelet counts. This relatively rare phenomenon has been found in the average population and is associated with some disease entities. It occurs with an incidence of approximately 0.1% of the average population. It has never been reported to be associated with a bleeding tendency or dysfunction of platelets[3].
References
[1] Zahed Mohammadi, Hamid Jafarzadeh, Sousan Shalavi. “Ethylenediaminetetraacetic acid in endodontics.” European Journal of Dentistry 7 Suppl 1 (2013): S135–S142.
[2] Giuseppe Banfi, Giuseppe Lippi, Gian Luca Salvagno. “The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes.” Clinical chemistry and laboratory medicine 45 5 (2007): 565–76.
[3] Izhar Shabnam, Joshi B C, Chuphal D S. “Ethylenediaminetetraacetic Acid (EDTA) - dependent pseudothrombocytopenia: a case report.” JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH (2014): FL03-4.
- Related articles
- Related Qustion
- Ethylenediaminetetraacetic acid: Application, toxicity and environmental fate May 9, 2023
Ethylenediaminetetraacetic acid was developed by Franz Munz in Germany during the 1930s as an alternative to citric acid. EDTA is used as a food additive, in herbicides, and pharmaceuticals.
- The principal toxicity of EDTA Oct 18, 2021
Ethylenediaminetetraacetic acid (EDTA) was developed by Franz Munz in Germany during the 1930s as an alternative to citric acid. EDTA is used as a food additive, in herbicides, in pharmaceuticals, and in a variety of consumer products.
- Ethylenediaminetetraacetic acid(EDTA)– Physical Properties and Applications Apr 17, 2020
Edetic acid and EDTA. EDTA is white powder, which is soluble in sodium hydroxide, sodium carbonate and ammonia solution, and 160 parts of boiling water, and slightly soluble in cold water.
DIAD belongs to the azo compound, and it can be used to initiate the polymerization reaction between the polymer monomers because it‘s easy to decompose and form free radicals....
Apr 28,2024Organic reagentsn-Butyl Acetate is a clear, colorless liquid with a fruity odor. It is miscible with all conventional solvents such as alcohols, ketones, aldehydes, ethers, glycols, glycol ethers, aromatic hydrocarbons, and aliphatic hydrocarbons.....
Apr 28,2024Organic SolventsEthylenediaminetetraacetic acid
60-00-4You may like
Ethylenediaminetetraacetic acid manufacturers
- Ethylenediaminetetraacetic acid
-

- $10.00 / 1kg
- 2024-05-10
- CAS:60-00-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100tons
- Ethylenediaminetetraacetic acid
-

- $18.00 / 10kg
- 2024-05-09
- CAS:60-00-4
- Min. Order: 1kg
- Purity: 99.9
- Supply Ability: 5000
- Ethylenediaminetetraacetic acid
-

- $20.00/ kg
- 2024-05-06
- CAS:60-00-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000kg/Week