ETHYLENE OXIDE: Applications and accidents
May 22,2023
General description
Ethylene oxide is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.
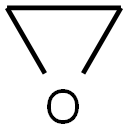
The reactivity that is responsible for many of ethylene oxide's hazards also make it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylene glycol, ethanolamines, simple and complex glycols, polyglycol ethers, and other compounds. Although it is a vital raw material with diverse applications, including the manufacture of products like polysorbate 20 and polyethylene glycol (PEG) that are often more effective and less toxic than alternative materials, ethylene oxide itself is a very hazardous substance. At room temperature it is a flammable, carcinogenic, mutagenic, irritating, and anaesthetic gas.[1] Its appearance is as follows:

Figure 1 Appearance of ETHYLENE OXIDE.
Applications
Ethylene oxide is one of the most important raw materials used in large-scale chemical production. Most ethylene oxide is used for synthesis of ethylene glycols, including diethylene glycol and triethylene glycol, that accounts for up to 75% of global consumption. Other important products include ethylene glycol ethers, ethanolamines and ethoxylates. Among glycols, ethylene glycol is used as antifreeze, in the production of polyester and polyethylene terephthalate (PET – raw material for plastic bottles), liquid coolants and solvents. The direct use of ethylene oxide accounts for only 0.05% (2004 data) of its global production. Ethylene oxide is used as a sterilizing agent, disinfecting agent and fumigant as a mixture with carbon dioxide (8.5–80% of ethylene oxide), nitrogen or dichlorodifluoromethane (12% ethylene oxide). It is applied for gas-phase sterilization of medical equipment and instruments, packaging materials and clothing, surgical and scientific equipment; for processing of storage facilities (tobacco, packages of grain, sacks of rice, etc.), clothing, furs and valuable documents.[2]
Ethylene oxide is one of the most commonly used sterilization methods in the healthcare industry because of its non-damaging effects for delicate instruments and devices that require sterilization, and for its wide range of material compatibility.[3] It is used for instruments that cannot tolerate heat, moisture or abrasive chemicals, such as electronics, optical equipment, paper, rubber and plastics. It was developed in the 1940s as a sterilant by the US military, and its use as a medical sterilant dates to the late 1950s, when the McDonald process was patented for medical devices.
Accidents
Ethylene oxide is extremely flammable, and its mixtures with air are explosive. When heated it may rapidly expand, causing fire and explosion. A number of industrial accidents have been attributed to ethylene oxide explosion. The autoignition temperature is 429 °C (804 °F), decomposition temperature of 571 °C (1,060 °F) at 101.3 kPa (14.69 psi), minimum inflammable content in the air is 2.7%,[4] and maximum limit is 100%. The NFPA rating is NFPA 704. Ethylene oxide in presence of water can hydrolyze to ethylene glycol and form poly ethylene oxide which then eventually gets oxidized by air and leads to hotspots that can trigger explosive decomposition. Fires caused by ethylene oxide are extinguished by traditional media, including foam, carbon dioxide or water. Suppression of this activity can be done by blanketing with an inert gas until total pressure reaches non explosive range. Extinguishing of burning ethylene oxide is complicated by that it can continue burning in an inert atmosphere and in water solutions. Fire suppression is reached only upon dilution with water above 22:1.[5]
References
[1]Rebsdat, Siegfried and Mayer, Dieter (2005) "Ethylene Oxide" in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_117.
[2]"Ethylene oxide". Chemical Backgrounders Index. The Environment Writer. Archived from the original on 28 August 2006. Retrieved 29 September 2009.
[3]"Ethylene Oxide Sterilization". Isometrix. Archived from the original on 2 April 2016.
[4]"Ethylene Oxide". Health and Safety Guide. International Programme on Chemical Safety (IPCS) INCHEM. 1988. Retrieved 23 September 2009.
[5]"Ethylene Oxide Safety Literature" (PDF). Shell Chemicals. Archived from the original (PDF) on 3 March 2016. Retrieved 23 October 2009.
- Related articles
- Related Qustion
- Uses and toxicity of ethylene oxide Oct 25, 2021
Ethylene oxide, also known as oxane, ethylene oxide, and ETO, it is a strong alkylating agent that can readily react with cellular nucleophiles to inactivate a variety of cellular macromolecules.
Ammonium lauryl sulfate is considered as environmentally friendly, as it is very quickly biodegradable and won’t typically cause any allergies.....
May 19,2023Catalyst and AuxiliaryBis(pinacolato)diboron is an important organic intermediate. In Suzuki reaction, it has the advantages of high reaction selectivity, mild conditions, and high yield.....
May 22,2023Organic Synthesis IntermediateETHYLENE OXIDE
75-21-8You may like
- How to synthesize Benzyl vinylcarbamate?
Mar 26, 2024
- Polypropylene and Polyvinyl chloride: Which one is better?
Mar 21, 2024
- How to synthesize Isoprene?
Mar 20, 2024
- OXANE
-

- $20.00 / 1KG
- 2023-10-24
- CAS:75-21-8
- Min. Order: 1KG
- Purity: 0.99
- Supply Ability: 20 tons
- ETHYLENE OXIDE
-

- $0.00 / 25KG
- 2023-08-30
- CAS:75-21-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- ETHYLENE OXIDE
-
- $1.10 / 1g
- 2023-07-27
- CAS:75-21-8
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons