Favipiravir: mechanism of action, pharmacokinetics and safety
Sep 22,2023
General Description
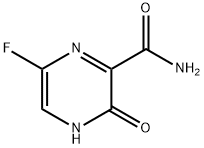
Favipiravir is a prodrug that is converted into an active form within cells. It inhibits RNA-dependent RNA polymerase (RdRp), disrupting viral replication and leading to chain termination and mutagenesis. It has a broad spectrum of effectiveness against various RNA viruses. Favipiravir-RTP, the active form, acts as a mutagen, increasing the frequency of mutations and reducing viral RNA and infectious particles. It binds strongly to RdRp and targets SARS-CoV-2. Favipiravir undergoes ribosylation and phosphorylation in tissues and is primarily metabolized by hepatic aldehyde oxidase. It exhibits nonlinear pharmacokinetics and can inhibit its own metabolism. Common side effects include gastrointestinal issues and increased liver enzymes. It is contraindicated in pregnant and lactating women and patients with severe hepatic or renal impairment. Caution is advised in patients with gout or hyperuricemia.

Figure 1. Tablets of favipiravir
Mechanism of action
Favipiravir is a prodrug that functions through a specific mechanism. It is converted into active favipiravir ribofuranosyl-5B-triphosphate (favipiravir-RTP) within cells by phosphoribosylation. This medication acts as a potent inhibitor of RNA-dependent RNA polymerase, which is essential for the replication of RNA viruses. The unique feature of favipiravir is its incorporation into viral RNA during synthesis by the error-prone viral RdRp. This incorporation disrupts the replication process, leading to chain termination and viral mutagenesis. Favipiravir's effectiveness spans across various types of RNA viruses due to the presence of RdRp in different strains. After being integrated into the viral RNA, favipiravir-RTP acts as a mutagen, evading repair mechanisms. This puts additional pressure on the nucleotide content of the coronavirus, particularly since SARS-CoV-2 already has a low cytosine level in its genome. As a result, favipiravir-RTP increases the frequency of mutations and exhibits a cytopathic effect. This effect contributes to the reduction in the quantity of viral RNA and infectious particles. Favipiravir demonstrates strong binding affinity to the RdRp complex, specifically targeting the Achilles heel of SARS-CoV-2. Its ability to inhibit viral replication makes it a potential therapeutic option for combating RNA viruses like SARS-CoV-2. 1
Pharmacokinetics
Favipiravir exhibits specific pharmacokinetic properties. Upon administration, it undergoes ribosylation and phosphorylation, leading to the formation of the activated metabolite favipiravir-RTP in tissues. The recommended dosing regimen involves 1600 mg on day 1, followed by 400 mg twice daily from day 2 to day 6, and then 400 mg once daily on day 7. The estimated area under the curve (AUC) for favipiravir is 1452.73 μg h/mL on day 1 and 1324.09 μg h/mL on day 7. The drug is primarily metabolized by hepatic aldehyde oxidase and partially by xanthine oxidase, resulting in the formation of an inactive oxidative metabolite. This metabolite is excreted in a hydroxylated form by the kidneys. Favipiravir demonstrates nonlinear pharmacokinetics and can self-inhibit its own metabolism via aldehyde oxidase inhibition, leading to increased circulating levels of the drug and its inactive metabolite. In individuals with mild to moderate hepatic impairment, there is a 1.4-1.8-fold increase in maximum plasma concentration (Cmax) and AUC after oral administration. Severe hepatic impairment results in a more substantial increase, with a 2.1-fold increase in Cmax and a 6.3-fold increase in AUC. Among patients with moderate renal impairment, there is a 1.5-fold increase in trough concentrations (Ctrough) compared to those with normal renal function. However, information regarding the use of favipiravir in patients with a glomerular filtration rate (GFR) below 30 mL/min is currently unavailable. 2
Safety
Favipiravir has been extensively studied and has a well-established safety profile based on the data from over 4000 patients. Common adverse events include gastrointestinal issues, elevated uric acid levels, decreased neutrophil count, increased liver enzymes, psychiatric symptoms, and increased blood triglycerides. The incidence of serious adverse events was 0.4%, and discontinuation due to adverse events was 1.1%. Similar rates of adverse events were observed with both low and high doses of favipiravir. Overall, favipiravir demonstrates a favorable safety profile. There are certain contraindications, precautions, and warnings associated with the use of favipiravir. It is contraindicated in pregnant and lactating women due to its potential teratogenic effects. Effective contraception should be used by both men and women of reproductive age during the treatment and for 7 days after therapy. It is also contraindicated in patients with hypersensitivity, severe hepatic impairment, and severe renal impairment. Caution should be exercised when administering favipiravir to patients with gout or a history of gout, as well as those with hyperuricemia. 3
Reference
1. Baranovich T, Wong SS, Armstrong J, Marjuki H, Webby RJ, Webster RG, Govorkova EA. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol. 2013 Apr;87(7):3741-3751.
2. Joshi S, Parkar J, Ansari A, Vora A, Talwar D, Tiwaskar M, Patil S, Barkate H. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021 Jan;102:501-508.
3. Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020 Apr 30;6(2):45-51.
- Related articles
- Related Qustion
- Favipiravir: A Repurposed Antiviral Drug Showing Promising COVID-19 Treatment Feb 19, 2024
Favipiravir shows promise in treating COVID-19 by inhibiting virus replication, with effectiveness dependent on a higher dosing regimen than for influenza.
- What is Favipiravir? Jul 21, 2020
Favipiravir is a pyrazine carboxamide derivative developed by Toyama Chemical of Japan to act against many RNA viruses. It was first described as a selective inhibitor of influenza virus replication with minimal cytotoxicity.
Altrenogest is a synthetic hormone used in veterinary medicine to control the reproductive cycle, primarily in female animals like pigs and horses.....
Sep 22,2023APIAntioxidant 245 is a versatile chemical with excellent antioxidant properties, used for long-term thermal stabilization and enhancing durability in various industries.....
Sep 22,2023APIFavipiravir
259793-96-9You may like
- Favipiravir
-

- $280.00 / 100kg
- 2024-06-03
- CAS:259793-96-9
- Min. Order: 100kg
- Purity: 99.99%
- Supply Ability: 100Tons
- Favipiravir
-

- $0.00 / 1kg
- 2024-05-13
- CAS:259793-96-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000kg
- Favipiravir
-

- $0.00 / 25kg
- 2024-04-12
- CAS:259793-96-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000ton