Introduction of Deuterium oxide
May 11,2023
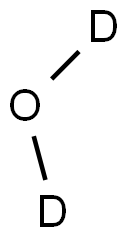
Deuterium oxide (D2O) is basically water composed of deuterium. It is also known as Heavy water. Deuterium is the hydrogen isotope with a mass double that of ordinary hydrogen. (Ordinary water is represented by H2O.) Deuterium oxide has a molecular weight of about 20 (the sum of twice the atomic weight of deuterium, which is 2, in addition to the atomic weight of oxygen that is 16), whereas ordinary water has a molecular weight of about 18 (twice the atomic weight of ordinary hydrogen, which is 1, plus oxygen, which is 16).
Ordinary water can be found mainly from renewable natural resources which include about one deuterium atom for every 6,760 ordinary hydrogen atoms. Therefore, the residual water contains a good quantity of deuterium content. To get deuterium oxide, continued electrolysis of hundreds of litres of water until only a few millilitres remain. Only then, D2O can be considered as almost pure. Until 1943, the only large-scale method used to produce Deuterium oxide was continuous electrolysis. It has been superseded by less expensive processes, such as fractional distillation. Fractional distillation is effective because D2O becomes concentrated in the liquid residue since it is less volatile than H2O. One major use of Deuterium oxide is that it is used as a moderator of neutrons in nuclear power plants. Deuterium oxide is employed as an isotopic tracer in studies of biochemical and chemical processes, in the laboratory.
Uses of Deuterium oxide
India is one of the world’s major manufacturers of Deuterium oxide. It exports D2O to countries like The United States and The Republic of Korea through its Deuterium oxide Board (HWB). The diverse applications and uses of Deuterium oxide are as follows:
Nuclear Magnetic Resonance: Since the signal from H2O solvent molecules interact and block the signal from the molecule of interest, D2O is used. D2O has a different magnetic moment and hence makes it convenient to be used in nuclear magnetic resonance spectroscopy.
In Organic Chemistry: Specifically labelled isotopologues of organic compounds require deuterium for their preparation. D2O is often used as a source for Deuterium.
Neutron Moderator: D2O is used as a neutron moderator in certain types of nuclear reactors. It helps in slowing down neutrons so that the probability of them reacting with the fissile uranium-235 than with uranium-238 increases. This, in turn, helps in capturing neutrons without fissioning.
Neutrino Detector: Neutrinos react with Deuterium oxide to produce flashes of lights.
Metabolic Rate Testing in Physiology and Biology: Conducting tests to check the safety of mean metabolic rate in humans and animals requires Deuterium oxide as part of a mixture with H218O.
Tritium Production: Tritium is created in small amounts in Deuterium oxide moderated reactors.
Preparation of Deuterium oxide
1) By Prolonged Electrolysis
When ordinary water is electrolysed, protium is formed. The liberation of protium happens readily in the case of H2O because H+ ions have greater mobility as compared to D+ ions. This factor owes to its smaller size. They also possess lower discharge potential and hence H+ ions are discharged at the cathode more easily. In addition to that, hydrogen atoms form molecular hydrogen more readily than deuterium atoms.
The concentration of Deuterium oxide in ordinary water increases as the electrolysis continues. Eventually, almost pure Deuterium oxide can be obtained when very little volume remains.
Brown, Degget and Urey designed an electrolytic cell for the preparation of Deuterium oxide. It consists of a steel cell 45 cm long and 10 cm in diameter. This is the cathode. A large number of these cells are used for the electrolysis of water in different stages.
2) By Fractional Distillation
There is a small difference in the boiling points of normal and Deuterium oxide. This is the basis of using fractional distillation for the preparation of heavy water. The boiling point of normal water is 373K whereas that of heavy water is 374.42K (at normal atmospheric pressure).
- Related articles
- Related Qustion
Orlistat is an internationally recognized new form of weight loss drug. Its commercial name is Sainike and first went on sale in New Zealand in 1998.....
May 11,2023DrugsTantalum(V) chloride is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry.....
May 15,2023Organic ChemistryDEUTERIUM OXIDE
7789-20-0You may like
- Crystal Structure of Aluminum Selenide
Apr 23, 2024
- Crystal Structure and Property of Tin Selenide
Apr 23, 2024
- Crystal Structure of Uranium Carbide
Apr 22, 2024
- DEUTERIUM OXIDE
-
- $100.00 / 1KG
- 2023-12-24
- CAS:7789-20-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- DEUTERIUM OXIDE
-

- $15.00 / 1KG
- 2023-03-04
- CAS:7789-20-0
- Min. Order: 1KG
- Purity: 99.9%
- Supply Ability: 10 Ton
- DEUTERIUM OXIDE
-

- $15.00 / 1KG
- 2021-07-10
- CAS:7789-20-0
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton