L(-)-Carvone: An Comprehensive Review on Health Benefits and Pharmacological Properties
Apr 7,2023
General Description
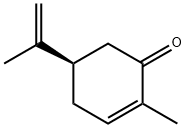
L(-)-Carvone is a monoterpene ketone (R)-2-Methyl-5-(prop-1-en-2-yl)cyclohex-2-enone (C10H14O) with a boiling point of 230 ◦C, which has an asymmetric carbon. It is synthetized and secreted as a secondary metabolite from essential oils, and its major role in the plant remains unclear. Currently, many studies have proven that carvone has promising pharmacological properties. This molecule has also demonstrated an antidiabetic effect, through its role in the prevention of obesity and metabolic problems associated with high-fat diets, achieved by improving glycoprotein component abnormalities and controlling glucose metabolism. The use of L(-)-Carvone as an antifungal has also been investigated against various fungi strains (Candida spp.), mycotoxigenic fungi (Fusarium spp., Aspergillus spp., and Penicillium spp.), and dermatophytes (Trichophyton spp., Epidermophyton floccosum, and Microsporum spp.). Additionally, L(-)-Carvone can be usedas an antibacterial agent against many strains of bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). It also exhibited an antibiofilm effect against S. aureus. On the other hand, carvone had anticancer activity against different cancer cell lines, including myeloma and melanoma cells, and breast cancer cells. All these properties have enabled the use of carvone in other fields, such as the disinfection of food packaging and medical devices.1-3

Figure 1. Properties of L(-)-Carvone
Pharmacological Properties of L(-)-Carvone
Neurological Activity
Several authors have attempted to study the effect of the use of L(-)-Carvone on some neurological disorders such as depression, sedation, nociception, seizure, local anesthesia, as well as its effect on some receptors and on the action potential. To study these effects, different models are used such as Swiss mice, Wistar rats, frog's sciatic nerve, and cortical neurons prepared from the cerebral cortices of rat fetuses.4
Antidiabetic Activity
Several studies showed the antidiabetic effects of volatile compounds including L(-)-Carvone. Indeed, three separate studies were interested in the potential antidiabeti activity of carvone by revealing its overall activity on in vivo models such as C57BL/6 mice with high-fat diet-induced obesity and streptozotocin (STZ)-induced diabetes in Wistar rats. Those studies have also focused on the main underlying mechanism of action exhibited by this molecule following multiple biochemical, hematological, and histopathological analyses.5
Antibacterial Activity
The antibacterial activity of L(-)-Carvone has been studied by several authors against multiple strains such as Escherichia coli, S. aureus, Streptococcus faecalis, and Pseudomonas aeruginosa. Different enantiomers were used in comparative studies to assess the structure–function relationship. Some studies were interested in the antibacterial effect of the encapsulated form (carvone loaded PLGA nanoparticles), others in its microbial transformation, while others were interested in manufacture of carvone biofilms (antibacterial coating) to prevent bacterial colonization of medical devices.6
Anticancer Activity
Similar to several other bioactives which showed promising anticancer properties, L(-)-Carvone has also been studied for its anticancer properties. Indeed, several in vitro investigations based on cell culture tests showed that this compound exhibits antiproliferative effects against various cancer cell lines. In this sense, the results of the study conducted by Ding et al. demonstrated that L(-)-Carvone exerts significant antiproliferative effects against myeloma cancer cells in a dose-dependent manner. The anticancer activities were linked to the induction of apoptosis and the G2/M cell cycle arrest. Moreover, L(-)-Carvone could inhibit cell invasion and p-P38 protein expression at an IC50 of 20 µM. In another study, Gopalakrishnan et al. evaluated the chemopreventive efficacy of L(-)-Carvone (at 10, 20, and 30 mg/kg b.w.) in vivo using 7,12-dimethylbenz(a)anthracene (DMBA)-induced skin carcinogenesis. The results showed that the tumor incidence of 100% in DMBA-painted animals as well as L(-)-Carvone at a dose of 20 mg significantly prevented skin carcinogenesis. In addition, this study showed decreased levels of phase I enzymes (cytochrome P450 and cytochrome b5) with increased levels of phase II enzymes (GR, GST, and GSH) and increased expression of Bax, caspase-3, and caspase-9 with decreased expression of mutated p53 and Bcl-2 in animals treated with DMBA and L(-)-Carvone (20 mg).7
Natural Sources of L(-)-Carvone
L(-)-Carvone is the major compound of the essential oil (EO) of many species of the Lamiaceae family; Mentha, Mentha spicata, Mentha × villoso-nervata, Mentha piperita L., Mentha crispa L., and Mentha cardiaca L.. Moreover, L(-)-Carvone is the major compound of the EO of Anethum graveolens, Thymus vulgaris, Majorana hortensis, Carum carvi, Anethum sowa, and Solanum tuberosum L.. In addition, other species belonging to the Orchidaceae family have been characterized by their richness in L(-)-Carvone; Catasetum discolor, Catasetum longifolium, Catasetum integerrimu, Catasetum macroglossu, Catasetum tabular, Catasetum veracruz, Catasetum viridiflavu, and Lippia alba. Secondary metabolites in EOs are variable depending on certain factors, including geographic origin, stages of development, and parts of the plant. Indeed, some studies have proven this variability between geographic locations and suggest the fluctuation of these phytochemicals in plants for responding to environmental situations.8
Preparation of L(-)-Carvone
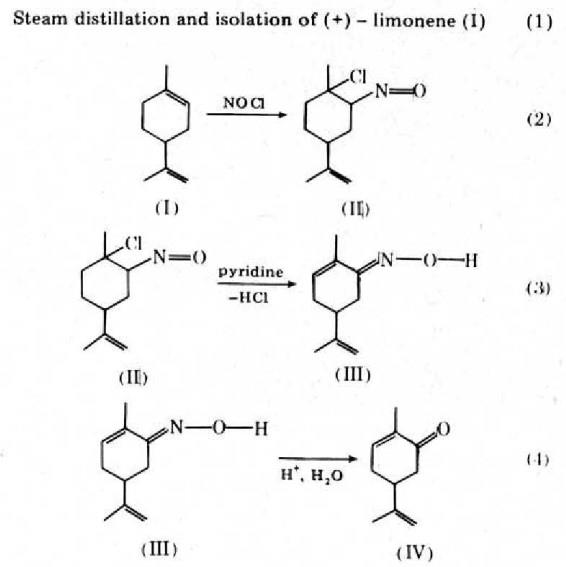
Figure 2. Preparation of L(-)-Carvone from (+)-Limonene
The first step in this sequence illustrates the isolation of a natural product by steam distillation. Isolation of the 10 g of (+)-limonene required for the second step demands approximately 25 oranges. The second step of the sequence, conversion of (+)-limonene into the limonene nitrosochloride (II), is an excellent example of regiospecific addition to a double bond. The addition is selective to the ring double bond and occurs in Markownikoff fashion. The third step of the sequence, conversion of the limonene nitrosochloride (II) into the carvoxime (III), is an example of an elimination. The reaction is run under reflux, and it is quite facile. The synthesis is completed by the conversion of the carvoxime into L(-)-Carvone (IV). This reaction is an example of the hydrolysis of a ketone derivative. The final product, L(-)-Carvone, is steam distilled from the reaction mixture and isolated by extraction with methylene chloride.9
References
1. Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.-E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional Use, Phytochemistry, Toxicology, and Pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318.
2. Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula stoechas Essential Oil from Morocco as Novel Source of Antileishmanial, Antibacterial and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184.
3. Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; El-Shazly, M.; Chamkhi, I. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Toxicology of Ajuga iva (L.) Schreb. J. Ethnopharmacol. 2020, 258, 112875.
4. De Sousa, D.P.; De Farias Nóbrega, F.F.; De Almeida, R.N. Influence of the Chirality of (R)-(-)- and (S)-(+)-Carvone in the Central Nervous System: A Comparative Study. Chirality 2007, 19, 264–268.
5. Alsanea, S.; Liu, D. BITC and S-Carvone Restrain High-Fat Diet-Induced Obesity and Ameliorate Hepatic Steatosis and Insulin Resistance. Pharm. Res. 2017, 34, 2241–2249.
6. Moro, I.J.; Gondo, G.D.G.A.; Pierri, E.G.; Pietro, R.C.L.R.; Soares, C.P.; de Sousa, D.P.; dos Santos, A.G. Evaluation of Antimicrobial, Cytotoxic and Chemopreventive Activities of Carvone and Its Derivatives. Braz. J. Pharm. Sci. 2018, 53.
7. Ayd?n, E.; Türkez, H.; Kele?s, M.S. Potential Anticancer Activity of Carvone in N2a Neuroblastoma Cell Line. Toxicol. Ind. Health 2015, 31, 764–772.
8. Mesa-Arango, A.C.; Montiel-Ramos, J.; Zapata, B.; Durán, C.; Betancur-Galvis, L.; Stashenko, E. Citral and Carvone Chemotypes from the Essential Oils of Colombian Lippia alba (Mill.) NE Brown: Composition, Cytotoxicity and Antifungal Activity. Mem. Inst. Oswaldo Cruz 2009, 104, 878–884.
9. Rothenberger, Otis S.; Krasnoff, Stuart B.; Rollins, Ronald B. (1980). "Conversion of (+)-Limonene to (?)-Carvone: An organic laboratory sequence of local interest". Journal of Chemical Education. 57 (10): 741.
- Related articles
- Related Qustion
- L(-)-Carvone: Multifaceted Compounds with Promising Therapeutic Potential Feb 6, 2024
L(-)-Carvone show antifungal, anti-tumor, and anti-inflammatory properties, making them potential candidates for controlling fungi, treating cancer, and managing inflammatory.
L(-)-Carvone
6485-40-1You may like
- L (-) -Carvone
-

- $120.00 / 1kg
- 2024-04-29
- CAS:6485-40-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- L(-)-Carvone
-

- $10.00/ kg
- 2024-04-25
- CAS:6485-40-1
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 10000ton
- L(-)-Carvone
-

- $0.00 / 1KG
- 2023-09-06
- CAS:6485-40-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg