Laboratory and medical uses of Sodium dodecyl sulfate (SDS) detergent
May 6,2024
What is Sodium dodecyl sulfate?
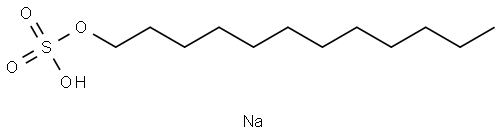
Sodium dodecyl sulfate (SDS), also known as Sodium Lauryl Sulfate (SLS), is an anionic surfactant that functions as a detergent, emulsifier and protein denaturant. It has a white to pale yellowpaste or liquid appearance with a slight odour. The chemical formula is C12H25O4SNa; SDS is an organic sodium salt of sulphate consisting of 12 carbon tails attached to a sulphuric acid group, giving the substance the amphiphilic properties required for detergents.SDS can be obtained by extraction from natural coconut or palm oil, or it can be prepared by chemical synthesis, whereby treatment of lauryl alcohol with sulphur trioxide gas, fuming sulphuric acid, or chlorosulphuric acid is employed to produce lauryl hydrogensulphate. The product is then neutralised by the addition of sodium hydroxide or sodium carbonate.

SDS has a wide range of uses in food, cosmetics and pharmaceuticals. It can be used as surfactants, emulsifiers, foaming agents, wetting agents, detergents, food additives, hair dyes and reagents for biochemical analysis. Especially in the production of personal care products, detergents and laboratory reagents.SDS is an active ingredient in many household cleaning and hygiene products, helping to reduce surface tension and enhance cleaning ability.
Laboratory Uses of SDS detergent
Sodium dodecyl sulfate (SDS) is an important tool in protein biochemistry research, and can be used to help lyse cells during DNA extraction and to unwind proteins in SDS-PAGE electrophoresis.The SDS-PAGE method involves denaturing proteins with the detergent SDS (breaking the non-covalent bonds in the proteins to denature them and cause them to lose their original shapes), and then pulling them through a polyacrylamide gel with an electric current in a process called polyacrylamide gel electrophoresis (PAGE). They are then pulled through a polyacrylamide gel with an electric current in a process called polyacrylamide gel electrophoresis (PAGE) SDS binds strongly to proteins, with approximately one detergent molecule binding to two amino acids at a SDS level of 0.1%. When boiled with SDS, proteins acquire a negative charge proportional to their molecular size and thus move through the acrylamide gel according to their molecular size. The smaller the size of the mobile protein, the faster it passes through the gel pores.
Aqueous SDS solutions are commonly used to disperse or suspend nanotubes. Another application is the analysis of haemoglobin. The hydrophobic groups of the detergent act on the globin subunits, causing a change in their conformation. The hydrophilic groups of SLS then bind to the iron oxide subunits to produce a stable reaction product, which can then be analysed to produce a haemoglobin value as part of a complete blood count.
Medical Uses of SDS detergent
SDS detergent is used medically as a rectal laxative in enemas and as an excipient in certain dissolvable aspirin and other fibre therapy capsules.SDS has also been proposed as a potential topical microbiocide for intravaginal use, to inhibit and potentially protect against viral infections, including Herpes Simplex Virus, HIV and Semliki Forest Virus.
- Related articles
- Related Qustion
- Sodium dodecyl sulfate: Application, preparation and toxicity Apr 20, 2023
Sodium dodecyl sulfate is an anionic surfactant used in many cleaning and hygiene products. It consists of a 12-carbon tail attached to a sulfate group.
- Sodium dodecyl sulfate : A very useful surfactant for Scientific Invetigations Nov 8, 2019
Sodium dodecyl sulfate is most researched and best understood widely used anionic surfactant. It is obtained in a state of powder as well as in pellet and used in Polymer Biotechnology and Biochemistry. This review article is rela
Sodium dodecyl sulfate
151-21-3You may like
Sodium dodecyl sulfate manufacturers
- Sodium dodecyl sulfate
-

- $6.00 / 1KG
- 2024-05-27
- CAS:151-21-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20TONS
- Sodium dodecyl sulfate
-

- $0.00 / 25kg
- 2024-05-15
- CAS:151-21-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons
- Sodium dodecyl sulfate
-

- $0.00 / 25kg
- 2024-05-15
- CAS:151-21-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons