Laccase: Overview, Production, Mechanism of Action, Uses in Food Processing etc.
Mar 11,2022
Laccases are multicopper oxidases found in plants, fungi, and bacteria. Laccases oxidize a variety of phenolic substrates, performing one-electron oxidations, leading to crosslinking. For example, laccases play a role in the formation of lignin by promoting the oxidative coupling of monolignols, a family of naturally occurring phenols. Other laccases, such as those produced by the fungus Pleurotus ostreatus, play a role in the degradation of lignin, and can therefore be classed as lignin-modifying enzymes. Other laccases produced by fungi can facilitate the biosynthesis of melanin pigments. Laccases catalyze ring cleavage of aromatic compounds.
Laccase EC 1.10.3.2, a glycoprotein, is an extracellular multicopper enzyme and is considered as a metal. Laccase is widely distributed in fungi and also found among the higher plants, bacteria and insects.
Laccases play an important role in food industry, paper and pulp industry, textile industry, synthetic chemistry, cosmetics, soil bioremediation and biodegradation of environmental phenolic pollutant and removal of endocrine disruptors. These enzymes are used for pulp delignification, pesticide or insecticide degradation, organic synthesis, waste detoxification, textile dye transformation, food technological uses, and biosensor and analytical applications.
Recently laccases have been efficiently applied to nanobiotechnology due to their ability to catalyze electron transfer reactions without additional cofactor. The technique for the immobilization of biomolecule such as layer-by-layer, micropatterning, and self-assembled monolayer technique can be used for preserving the enzymatic activity of laccases.
Sources
Laccase is generally found in higher plants and fungi but recently it was found in some bacteria such as S.lavendulae, S.cyaneus, and Marinomonas mediterranea. In fungi, laccases appear more than the higher plants. Basidiomycetes such as Phanerochaete chrysosporium, Theiophora terrestris, and Lenzites, betulina, and white-rot fungi such as Phlebia radiate, Pleurotus ostreatus, and Trametes versicolour also produce laccase. Many Trichoderma species such as T. atroviride, T. harzianum, and T. longibrachiatum are the sources of laccases. Laccase from the Monocillium indicum was the first laccase to be characterized from Ascomycetes which shows peroxidase activity. Pycnoporus cinnabarinus produces laccase as ligninolytic enzyme while Pycnoporus sanguineus produces laccase as phenol oxidase. In plants, laccase plays a role in lignifications whereas in fungi it has been implicated in delignification, sporulation, pigment production, fruiting body formation, and plant pathogenesis.
Properties of Laccase Enzyme
Laccases are mainly monomeric, dimeric, and tetrameric glycoprotein. Glycosylation plays an important role in copper retention, thermal stability, susceptibility to proteolytic degradation, and secretion. Upon purification, laccase enzymes demonstrate considerable heterogeneity. Glycosylation content and composition of glycoprotein vary with growth medium composition.
Applications
Laccases are multicopper-oxidases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) that are able to oxidize phenolic substrates (e.g. 2,6-dimethoxy-phenol), aromatic amines (e.g. 1-hydroxybenzotriazole), or polycyclic aromatic hydrocarbons (e.g. anthracene) . The oxidation of the substrate occurs via a oneelectron reduction and is accompanied by a reduction of molecular oxygen to water.
Most laccases are of fungal origin, but they also occur in bacteria, insects, and plants. Due to the broad substrate range of laccases, their possible industrial usage is widespread. Nevertheless, only few applications have been commercialized up to now, mostly in the textile industry. In the food and feed sector, laccases have been evaluated for different applications, such as the stabilization of beverages, the reduction of off-flavors, the improvement of wheat dough, and the usage of laccases as biosensors in the food processing industry. Off-flavors in wine may occur due to microbial conversion of phenolic compounds present in the wine itself or in the cork stoppers.
The polymerization reactions catalyzed by laccases can also be used to improve the shade of food, such as the coloration of tea-based products. In the bakery industry, laccase might be used together with proteases or xylanases to improve the dough quality. It was proposed that laccases oxidize ferulic acid attached to the arabinoxylan present in cereal flour. The obtained phenolic radicals can undergo a nonenzymatic reaction, resulting in cross-linked feruloylated arabinoxylans. In oat flour-based bread, the usage of laccase increased the loaf-specific volume and decreased the crumb hardness. Contradictory, in another study, laccase alone decreased the specific volume and increased the crumb hardness. A combined usage of laccase with xylanase improved again the oat flour bread properties.
Applications of Laccase in Food Processing
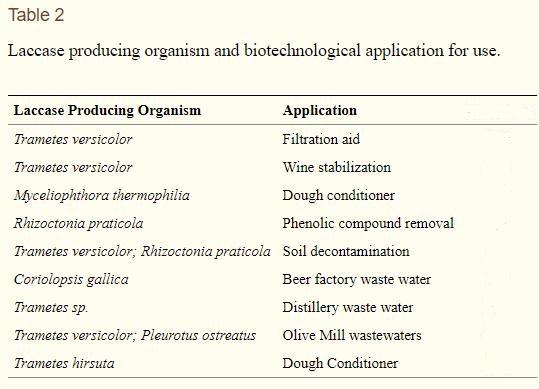
Table 2 shows the multiple applications of laccases in the food industry. Areas of the food industry that benefit from processing with laccase enzymes include baking, juice processing, wine stabilization, and bioremediation of waste water. The use of laccase enzymes allows for the improvement of functionality along with sensory properties. Laccase can also be utilized for analytical applications including biosensors, enzymatic, and immunochemical assays.
Mechanism of Laccase Action
The catalysis of laccase occurs with reduction of one molecule of oxygen to water accompanied with one electron oxidation of a wide range of aromatic compounds which includes polyphenols, methoxy-substituted monophenols, and aromatic amines. This oxidation results in generation of oxygen-centered free radical that can be converted to quinone in a second enzyme catalyzed reaction. Laccase catalysis occurs in three steps: (1) type I Cu reduction by substrate; (2) electron transfer from type I Cu to the type II Cu and type III Cu trinuclear cluster; (3) reduction of oxygen to water at the trinuclear cluster.
The laccase mediated catalysis can be extended to nonphenolic substrates by the inclusion of mediators. Mediators are a group of low molecular weight organic compounds that can be oxidized by laccase first forming highly active cation radicals capable of oxidizing nonphenolic compounds that laccase alone cannot oxidize (Figure 1). The most common synthetic mediators are 1-hydro-xybenzotriazole (HOBT), N-hydro-xyphthalimide (NHPI), and 2,2′-azinobis-3-ethylthiazoline-6-sulfonat (ABTS).
- Related articles
- Related Qustion
Metronidazole is a nitroimidazole drug similar to tinidazole. It has the chemical formula 1-(2-hydroxyethyl)- 2-methyl-5-nitroimidazole (C6H9N3O3) and a low molecular weight of 171.15 kDa. Following the discovery that azomycin, a nitroimida....
Mar 10,2022APIIn spite of the availability of many new antibiotics, and the progressive development of resistance in bacterial species, penicillin G (Pen G) remains a very effective agent.....
Mar 11,2022APILaccase
80498-15-3You may like
- Potassium carbonate: a food additive
Apr 15, 2024
- Can Ammonium bicarbonate be used as a food additive?
Apr 9, 2024
- How to Melt Coconut Oil at Home?
Mar 21, 2024