Non-polar molecule with polar bonds: CO2
Dec 19,2023
Definition of Polarity
Polarity is a physical property of compounds related to other physical properties such as melting and boiling points, solubility, and intermolecular interactions between molecules. The polarity of a bond arises from the relative electronegativities of the elements. Electronegativity is the power of an atom of an element to attract electrons toward itself when it is part of a compound. Thus, although a bond in a compound may consist of a shared pair of electrons, the atom of the more electronegative element will draw the shared pair toward itself and thereby acquire a partial negative charge. The atom that has lost its equal share in the bonding electron pair acquires a partial positive charge because its electrons no longer fully cancel its nuclear charge.
Polar Molecule vs Non- Polar Molecule
The bond or the molecular polarities depend upon the electronegativities of the atoms or the molecules. A molecule is said to be either a polar, nonpolar, or ionic molecule.
Polar Molecules
A polar molecule is usually formed when one end of the molecule is said to possess more positive charges, whereas the opposite end of the molecule has negative charges, creating an electrical pole. When a molecule is said to have a polar bond, then the center of the negative charge will be on one side, whereas the center of the positive charge will be on a different side. The entire molecule will be polar.
Non- Polar Molecules
A molecule that does not have the charges present at the end because electrons are finely distributed, and those that symmetrically cancel out each other are nonpolar molecules. A polar molecule cannot be mixed with a nonpolar molecule in a solution. For example, consider water and oil. Water is a polar molecule in this solution, whereas oil behaves as a nonpolar molecule. These two molecules do not form a solution as they cannot be mixed.
Is carbon dioxide a polar molecule?
For the most part, there is a direct correlation between the polarity of a molecule and the number and types of polar or nonpolar covalent bonds present. In a few cases, a molecule may have polar bonds but in a symmetrical arrangement, giving rise to a nonpolar molecule such as carbon dioxide.
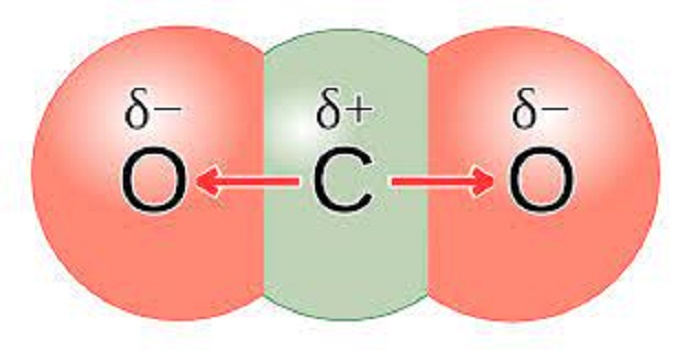
Carbon dioxide is nonpolar because it has a linear, symmetrical structure, with 2 oxygen atoms of equal electronegativity pulling the electron density from carbon at an angle of 180 degrees from either direction. As shown below, these double bonds are 180 degrees from the central carbon atom. Therefore, as the oxygen atom on the right tries to pull the electron density from the carbon over itself, the (other) oxygen atom, i.e., the one on the left, pulls the electron density over itself with equal force. Polarity in a molecule occurs due to the unequal sharing of valence electrons; since there is no unequal sharing of valence electrons in the case of carbon dioxide, it is nonpolar.
- Related articles
- Related Qustion
- Dry ice:Chemical formula,Uses,Safety Mar 19, 2024
Dry ice is the solid form of carbon dioxide (chemical formula CO2), comprising two oxygen atoms bonded to a single carbon atom.
- Is carbon dioxide a mixture or a pure substance? Feb 29, 2024
Carbon dioxide (CO2) is indeed considered a pure substance. A molecule of carbon dioxide (CO2) is made up of one carbon atom and two oxygen atoms.
- The Lewis structure of Carbon dioxide Nov 8, 2023
The carbon-oxygen ratio in a CO2 molecule is 1:2. Two double bonds connect the carbon and oxygen atoms in the Lewis structure.
NaCl is a chemical formula for Sodium Chloride, and it is made of one Sodium atom and one chlorine atom. The atoms of both Sodium and Chlorine are arranged in an organized pattern, also known as a lattice.....
Dec 19,2023APILinolenic acid exerts positive effects on cardiovascular health by influencing arrhythmogenesis and thrombosis. Efficiently absorbed and distributed, it may lower the risk of CVD events and mortality.....
Dec 20,2023APICarbon dioxide
124-38-9You may like
- Crystal Structure of Aluminum Selenide
Apr 23, 2024
- Crystal Structure and Property of Tin Selenide
Apr 23, 2024
- Crystal Structure of Uranium Carbide
Apr 22, 2024
- CARBON DIOXIDE USP/EP/BP
-

- $1.10 / 1g
- 2021-07-29
- CAS:124-38-9
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min