PLX3397 (Pexidartinib)
Dec 11,2019
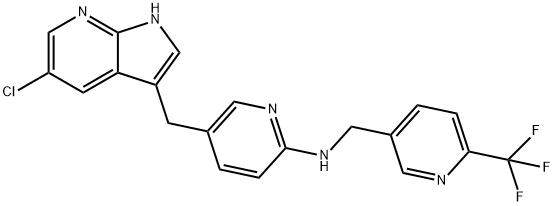
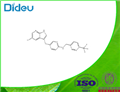
The IUPAC name of Pexidartinib (C20H15ClF3N5) is 5-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}methyl)-N-{[6-(trifluoromethyl)pyridin-3-yl]methyl}pyridin-2-amine, with the CAS registry number 1029044-16-3.
Pexidartinib (TURALIO™) is a novel, orally available small molecule tyrosine kinase inhibitor (TKI) with potent and selective activity against the colony-stimulating factor 1 (CSF1) receptor.1 Pexidartinib also inhibits KIT proto-oncogene receptor tyrosine kinase (KIT) and FMS-like tyrosine kinase 3 harboring an internal tandem duplication mutation (FLT3-ITD)2. Pexidartinib has been developed by Daiichi Sankyo for the treatment of tenosynovial giant cell tumor (TGCT; also known as giant cell tumor of the tendon sheath or pigmented villonodular synovitis3.
Pexidartinib 200 mg capsules were approved in the USA on 2 August 2019 for the treatment of adult patients with symptomatic TGCT associated with severe morbidity or functional limitations and not amenable to improvement with surgery4. Pexidartinib is the first systemic treatment to be approved by the US FDA in this indication. Approval was based on data from a pivotal phase III study (ENLIVEN), which constituted the first placebo-controlled trial of a systemic investigational treatment in TGCT. The recommended dose of pexidartinib is 400 mg administered twice daily on an empty stomach (≥1 h before or ≥2 h after a meal or snack). Capsules should be swallowed whole, rather than opened, broken or chewed. Treatment should continue until disease progression or unacceptable toxicity5.
The most common treatment emergent adverse events (TEAEs) of any grade (occurring in ≥15% of pexidartinib recipients and more than with placebo) were hair color changes, fatigue, increased aspartate aminotransferase (AST), increased alanine aminotransferase (ALT), dysgeusia, vomiting, periorbital edema, abdominal pain, decreased appetite, pruritus, hypertension and increased alkaline phosphatase (ALP). The most common grade 3 or 4 TEAEs (occurring in ≥5% of pexidartinib recipients) were increased ALT, increased AST, increased ALP and hypertension. TEAEs led to permanent treatment discontinuation in 13% of pexidartinib recipients; in most cases, discontinuation was due to liver-related TEAEs. Serious adverse events were reported in 13% of pexidartinib recipients during part one of ENLIVEN5.
The synthetic routes of pexidartinib were developed by Daiichi Sankyo Company6. A novel efficient synthetic route for pexidartinib was designed via Tsuji–Trost/Heck tandem reaction in combination with palladium and silver catalysis, which avoids the need for ultra-low temperature and greatly reduces the amount of corrosive trifluoroacetic acid7. The cheap and easily available materials and reagents and easy operations for workup and purification make this route more practical.
References
1.Ao, J. Y.; Zhu, X. D.; Chai, Z. T.; Cai, H.; Zhang, Y. Y.; Zhang, K. Z.; Kong, L. Q.; Zhang, N.; Ye, B. G.; Ma, D. N.; Sun, H. C., Colony-Stimulating Factor 1 Receptor Blockade Inhibits Tumor Growth by Altering the Polarization of Tumor-Associated Macrophages in Hepatocellular Carcinoma. Molecular cancer therapeutics 2017, 16 (8), 1544-1554.
2.Burton, E.; Wong, B.; Zhang, J. Z.; West, B.; Bollag, G.; Habets, G.; Galanis, A.; Nguyen, H.; Arowojolu, O.; Rajhowa, T.; Levis, M. J., The Novel Inhibitor PLX3397 Effectively Inhibits FLT3-Mutant AML. Blood 2011, 118 (21), 1551-1552.
3.Daiichi Sankyo Inc., TURALIOTM (pexidartinib) capsules, for oral use: US prescribing information 2019. https://www.accessdata .fda.gov/ 2019.
4.Lamb, Y. N., Pexidartinib: First Approval. Drugs 2019, 79 (16), 1805-1812.
5.Tap, W. D.; Gelderblom, H.; Palmerini, E.; Desai, J.; Bauer, S.; Blay, J. Y.; Alcindor, T.; Ganjoo, K.; Martin-Broto, J.; Ryan, C. W.; Thomas, D. M.; Peterfy, C.; Healey, J. H.; van de Sande, M.; Gelhorn, H. L.; Shuster, D. E.; Wang, Q.; Yver, A.; Hsu, H. H.; Lin, P. S.; Tong-Starksen, S.; Stacchiotti, S.; Wagner, A. J.; investigators, E., Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet 2019, 394 (10197), 478-487.
- Related articles
- Related Qustion
- How to synthesize PLX3397 (Pexidartinib)? Jan 22, 2024
?Pexidartinib (PLX3397) is a novel, orally available small molecule tyrosine kinase inhibitor (TKI) with potent and selective activity against the colony-stimulating factor 1 (CSF1) receptor.
- What is PLX3397 (Pexidartinib)? Feb 24, 2020
PLX3397 (Pexidartinib) is a small-molecule, oral, potent multi-targeted receptor tyrosine kinase (RTK) inhibitor of CSF-1R, Kit, and Flt3 and it can cross the blood-brain barrier. In M-NFS-60, Bac 1.2F5 and M-07e cells.
- Uses and Side effects of Pexidartinib Nov 4, 2019
Daiichi Sankyo’s pexidartinib has become the first FDA-approved therapy for tenosynovial giant cell tumour (TGCT), a rare form of tumour affecting joints and tendons. TGCT – which is also known as pigmented villonodular synovitis (PVNS) or
Methyl nonafluorobutyl ether is mainly used as a lubricant of high technology equipment. The ozone depletion potential (ODP) of methyl nonafluorobutyl ether is zero because it does not contain chlorine.....
Dec 11,2019Organic ChemistryFluocinolone acetonide is a fluorinated steroid that is flunisolide in which the hydrogen at position 9 is replaced by fluorine. A corticosteroid with glucocorticoid activity.....
Dec 12,2019Hormones and the Endocrine SystemPLX3397 (Pexidartinib)
1029044-16-3You may like
PLX3397 (Pexidartinib) manufacturers
- Pexidartinib
-

- $40.00 / 5mg
- 2025-09-17
- CAS:1029044-16-3
- Min. Order:
- Purity: 99.21%
- Supply Ability: 10g
- PLX3397 (Pexidartinib) USP/EP/BP
-
- $1.10 / 1g
- 2021-08-18
- CAS:1029044-16-3
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
- PLX3397 (Pexidartinib)
-

- $15.00 / 1KG
- 2021-07-13
- CAS:1029044-16-3
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton