Preparation of 9-(3-Bromophenyl)-9-phenyl-9H-fluorene
Nov 8,2019
9-(3-Bromophenyl)-9-phenyl-9H-fluorene (9-(3-bromophenyl)-9-phenylfluorene) is an important organic intermediate to synthetize its substituted products.
9-(3-Bromophenyl)-9-phenyl-9H-fluorene can be prepared according to the reported literatures [1-3].
Method 1
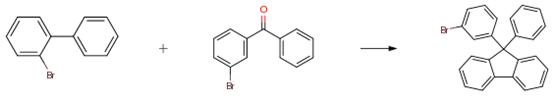
A solution of 10.0 g of 2-bromobiphenyl (42.9 mmol) in 70 ml of anhydrous THF was added to a 500 ml 3-neck flask and cooled to -78° C. 27 ml of a 1.58 M hexane solution of n-BuLi (42.9 mmol) was then added dropwise while stirring, and the reaction was stirred for about 2.5 hours. A solution of 9.30 g (35.6 mmol) of 3-bromobenzophenone in 85 ml of an anhydrous THF was added dropwise, and the resulting mixture was stirred for about 2 hours at -78° C. followed by stirring for about 3 hours at room temperature. 1 N hydrochloric acid (HC1) was then added to the mixture, and the mixture was stirred for about 1 hour. The mixture was washed with water, and the resultant organic phase was concentrated to afford a material having candy-like consistency. The candy-like material, 50 ml of acetic acid, and 2.4 ml of hydrochloric acid were added to a 500 ml recovery flask, and the mixture was stirred and reacted at 130° C. under a nitrogen atmosphere for about 2 hours. After the reaction, the reaction mixture was added dropwise to 350 ml of ice-cold water, precipitating white crystals, and the crystals were isolated by filtration. The crystals were washed with methanol and allowed to dry. About 13.3 g of the product was obtained as white powder (yield: about 78percent).
Method 2
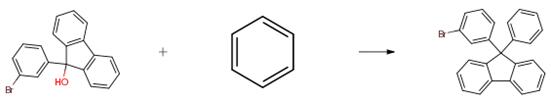
The 12.0 g (35.5 mmol) of starting material is put into a 250 ml round flask, then dissolved in anhydrous benzene solution. In 30 ml benzene dilution in three trifluoromethane sulfonic acid (6.3 ml, 2 eq) later, the diluted solution is slowly added to the reactant. The nitrogen flow heating and reflux obtained reactant 48 hours. For 0.5 N aqueous sodium hydroxide solution and water washing the reactant, under reduced pressure after appropriate except benzene, methanol (benzene 5 times) by adding wherein in order to obtain the crystalline solid, then filtering the crystalline solid to obtain compound I - 2 (8.5 g, 60 percent yield).
Method 3

In a 200-mL three-neck flask, 30 mL of a dehydrated THF solution of 4.2 g (18 mmol) of 2-bromobiphenyl was added thereto, and then the mixture solution was stirred at -78 0C. 11 mL (18 mmol) of an n-BuLi hexane solution (1.57 M) was dropped into this mixture solution, and the mixture was stirred for 2.5 hours. After that, 40 mL of dehydrated THF solution of 3.9 g (15 mmol) of 3-bromobenzophenone was dropped to this mixture, and the mixture was stirred for 2 hours and at room temperature for 16 hours. After the reaction, l M diluted hydrochloric acid was added to this mixture solution, and the mixture was stirred for 1 hour. This mixture was washed with water. The obtained organic phase was concentrated to obtain a candy-like substance. Then, in a 200-mL recovery flask, this candy-like substance, 20 mL of glacial acetic acid, and 1.0 mL of hydrochloric acid were put, and the mixture was heated and stirred under a nitrogen atmosphere at 130 0C for 2 hours to be reacted. After the reaction, this reaction mixture solution was dropped into 150 mL of ice-cooled water, so that a caramel-like solid was precipitated.
An insoluble component of this was removed by decantation. This caramel-like solid was dissolved in 100 mL of toluene, and a saturated sodium hydrogen carbonate aqueous solution was added thereto with stirring the toluene solution until no more bubble comes out. An organic layer of this was washed with water, and then silica gel was added to adsorb moisture. The filtrate which was obtained by filtration of the mixture was concentrated, and methanol was added thereto. The mixture was irradiated with ultrasonic wave while being cooled with ice and then the produced solid was filtrated. 4.9 g of an objective white powder was obtained at a yield of 83 percent.
References
1.Samsung Display Co., Ltd. Itoi H. Material for Organic Electroluminescent Device and Organic Electroluminescent Device Including the Same. US2016/359113[P], 2016, A1, Paragraph 0069; 0070.
2.Cheil Industries Co. Ltd. Ryu DW. Jo YG. Jong SH. Shin CJ. Lee HI. Kang US, Yu US, Jo PS. Organic compounds, organic photoelectric device and display device (by machine translation). KR2015/130221[P], 2015, A, Paragraph 0378-0380.
3.Semiconductor Energy Laboratory Co., Ltd. Osaka H, Shitagaki S, Suzuki T, Ohsawa N, Kawakami S, Seo S. Fluorene derivative, light-emitting element, light-emitting device, electronic device, and lighting device. WO2010/137601[P], 2010, A1, Page column 108-110.
- Related articles
- Related Qustion
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy is a heterocyclic compound, which is a 4-substituted 2,2,6,6-tetramethylpiperidyl-1-oxy (TEMPO) derivative. Like the related TEMPO, it is used as a catalyst and chemical oxidant.....
Nov 8,2019Catalyst and AuxiliaryCarbon is unique in its chemical properties because it forms a number of components superior than the total addition of all the other elements in combination with each other.....
Nov 8,2019Chemical Reagents9-(3-Bromophenyl)-9-phenyl-9H-fluorene
1257251-75-4You may like
9-(3-Bromophenyl)-9-phenyl-9H-fluorene manufacturers
- 9-(3-Bromophenyl)-9-phenyl-9H-fluorene
-

- $0.00 / 1KG
- 2024-01-23
- CAS:1257251-75-4
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 150KG /month
- 9-(3-Bromophenyl)-9-phenyl-9H-fluorene
-

- $200.00 / 1KG
- 2024-01-02
- CAS:1257251-75-4
- Min. Order: 1KG
- Purity: 99%, 99.5% Sublimated
- Supply Ability: g-kg-tons, free sample is available
- 9-(3-Bromophenyl)-9-phenyl-9H-fluorene
-

- $0.00 / 1kg
- 2023-12-20
- CAS:1257251-75-4
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 30000 Kg




