Production process of Isophytol
Mar 13,2024
Production process of Isophytol
According to the different key intermediates in the preparation process, the industrial production processes of isophytol include pseudoionone route and linalool route. The pseudoionone route using Litsea cubeba oil as a raw material is a traditional method for preparation of isophytol. However, due to the limitation of natural resource of Litsea cubeba oil, the production scale is small and cannot meet the market demand. Currently, linalool route is the most widely used process for isophytol production in the world. Various starting materials have been used for linalool synthesis, including acetylene–acetone (Roche process), isobutylene–formaldehyde–acetone (BASF process), isoprene and natural resource turpentine.
Roche process for production of isophytol was developed by Hoffmann-La Roche Company in Switzerland, which enabled the company to take the lead in mastering the large-scale production technology of vitamin E in the world and occupy a leading position in the market for a long time. In the process, acetylene and acetone were used as starting materials and underwent more than ten steps of reactions via methyl butynol, methyl heptenone, dehydrolinalool, linalool, dehydronerolidol, nerolidol, farnesylacetone, phyton and dehydroisophytol to produce isophytol. Various intermediates and isophytol produced by the Roche process are of good qualities. The production operations are easy to control and no corrosion to production equipment occurs. Therefore, the Roche process has been adopted by the vast majority of isophytol manufacturers in the world for production of isophytol.
Figure 4.6 shows the reaction route from nerolidol to isophytol. Nerolidol undergoes a Carroll reaction with methyl acetoacetate in the presence of aluminum isopropoxide catalyst at a temperature of 170°C for 3 h. The molar ratio of nerolidol and methyl acetoacetate is 1:1.3, and the catalyst dosage is 4% based on the weight of nerolidol. After the completion of the reaction, the reaction solution is distilled under reduced pressure, and the fraction at a temperature of 125–126°C and a pressure of 1.33 mbar is collected. The yield of farnesyl acetone was 95.8% with a purity of 99.4%.
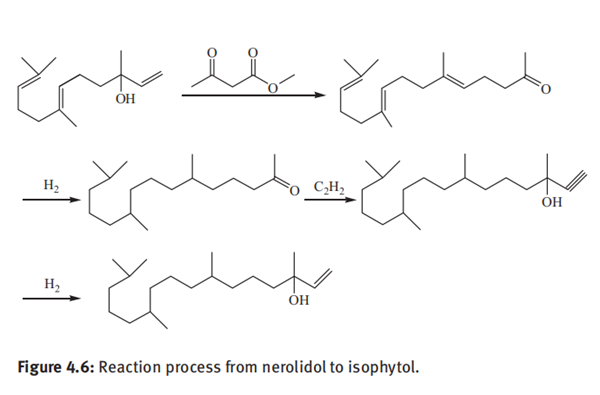
Farnesylacetone is dissolved in methanol and hydrogenated in the presence of Pd/C catalyst at room temperature under 0.141MPa for 24 h. After removal of the catalyst by filtration, the reaction solution is concentrated under reduced pressure to remove the solvent, and the residue is distilled under reduced pressure to collect a fraction at 152–153°C and 2.6 mbar. The yield of phyton is 95.4% with a purity of 99.2%.
Phyton underwent an ethynylation reaction with acetylene in potassium hydroxide/liquid ammonia system at 4–6 °C for 2.5h. An aqueous 25% ammonium sulfate was added to stop the reaction. The ammonia and unreacted acetylene were recovered. The dehydroisophytol was isolated by extraction with hexane and distillation. The conversion of phyton was 95.4%. The resultant dehydroisophytol was hydrogenated in the presence of a Lindlar catalyst under hydrogen pressure of 0.4 MPa and at a temperature between 30 and 60°C for 8h. After filtration of the catalyst and evaporation of the solvent, isophytol was obtained by vacuum distillation under 2–3 mmHg and collection of a fraction at 148–160°C. The yield of isophytol was 75.7% based on phyton.
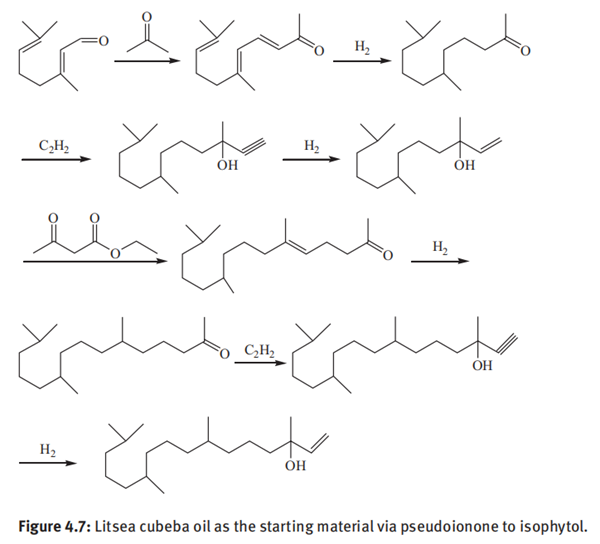
Preparation of isophytol from Litsea cubeba oil
Litsea cubeba oil is a kind of natural essential oil extracted from fresh fruit of Litsea cubeba, which is yellowish and contains more than 60% of citral. China is rich in Litsea cubeba oil resources which can be used to prepare isophytol. Figure 4.7 shows the reaction process using Litsea cubeba oil as the starting material via pseudoionone to isophytol. The citral in Litsea cubeba oil underwent a condensation reaction with acetone to yield pseudoionone. The resulting pseudoionone was hydrogenated and then reacted with acetylene to generate a C15 alkynol. Semihydrogenation of the C15 alkynol was followed by a Carroll reaction with ethyl acetoacetate leading to phyton. Ethynylation of the phyton with acetylene gave dehydroisophytol, which was partially hydrogenated to obtain isophytol.
- Related articles
- Related Qustion
Polyvinylpyrrolidone, or PVP, is one of the most essential fine chemicals derived from acetylene.....
Mar 13,2024Organic Chemistry2-Thiobarbituric acid is a versatile compound, effectively used in biochemistry and as an eco-friendly corrosion inhibitor for steel in oilfield environments.....
Mar 13,2024APIIsophytol
505-32-8You may like
- isophytol
-

- $0.00 / 1Kg
- 2024-04-08
- CAS:505-32-8
- Min. Order: 1Kg
- Purity: 99.9%
- Supply Ability: 200tons
- Isophytol
-

- $1.10 / 1g
- 2022-09-06
- CAS:505-32-8
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons Min
- Isophytol
-

- $10.00 / 1Kg/Bag
- 2021-11-16
- CAS:505-32-8
- Min. Order: 1Kg/Bag
- Purity: 99%
- Supply Ability: 20 Tons