Property, Synthesis, Function, and Bioactivities of 3-Hydroxytyramine Hydrochloride
Jul 12,2022
General description
The melting point (ºC) is 248-250 (lit.) [1], the molar refractive index is 43.10, the molar volume (cm3 /mol) is 122.7, the isotensional specific volume (90.2k) is 342.9 [3], the surface tension (DYNE /cm) is 60.8, and the polarization (10-24cm3) is 17.08 [4].
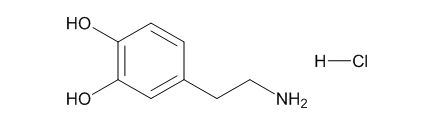
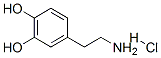
Fig. 1 The structure of 3-Hydroxytyramine hydrochloride
Toxicological data
Its acute toxicity reveals oral LD50 4361mg/kg(mus) 2859mg/kg(rat) [2]. It also irritates skin and mucous membranes. As for eyes, it shows the effects of stimulation. In addition, there is no known sensitization.
Synthesis
Fig. 2 The synthetic route of 3-Hydroxytyramine hydrochloride
Dissolve dopamine hydrochloride (160 mg) and n-dodecylthiol (40 μL) in a H2O/ethanol solution (20 mL, 1:1 v/v) at pH 8.5 under ultrasonication.
Immerse a piece of PU sponge (1.5 cm × 1.5 cm × 1.5 cm) in the resultant solution.
Stir for 10 h.
Rinse with water and dry at 80 °C to acquire the superhydrophobic PU sponge [5].
Identification
Take 0.1g of the product, add 5ml of water to dissolve, add 20ml of trinitrophenylphenol test solution, mix, stand to precipitate the crystals, and then filter. Rinse with water for crystallization and rinse with alcohol. Drying at 105 ℃, determined according to law, melting point is about 200 ℃, melting at the same time decomposition [6].
Take 10mg of the product, add 1ml of water to dissolve, then add 1 drop of ferric chloride test solution, the solution appears dark green. Then drop 1% ammonia solution, that is, turn purple.
Take this product and add 0.5% sulfuric acid solution to make a solution containing about 30μg per 1ml. According to spectrophotometry, it has the maximum absorption at the wavelength of 280nm [7].
The infrared absorption spectrum of the product should be consistent with that of the control (spectral set 345).
The aqueous solution of the product shows the identification reaction of chloride.
Content determination
Take about 0.15g of the product, weigh it accurately, add 25mL glacial acetic acid, boil and dissolve it, cool it to about 40℃, add 5mL mercury acetate test solution, cool it to room temperature, add 1 drop crystal violet indicator solution, titrate with perchloric acid (0.1mol/L) until the solution turns blue and green, and correct the titrated result with blank test. Each 1mL of perchloric acid titrant (0.1mol/L) corresponds to 18.96mg of C8H11NO2.HCl [8].
Functions and Uses
3-Hydroxytyramine hydrochloride is the precursor of norepinephrine biosynthesis and one of the central transmitters, which has the role of stimulating β -receptors, α -receptors and dopamine receptors. The stimulation of cardiac β -receptors can increase myocardial contractility and cardiac output. Excitatory dopamine receptor and α -receptor can dilate kidney, mesentery, coronary artery and cerebrovascular and increase blood flow. It has mild contraction effect on peripheral blood vessels and increases arterial blood pressure [9]. The prominent effect of this drug is to increase renal blood flow and increase glomerular filtration rate, thus promoting the increase of urine volume and urine sodium excretion. Clinical use of the drug is for various types of shock, especially for shock accompanied by weakened cardiac contractility, renal insufficiency [10].
Clinical preparations of 3-Hydroxytyramine hydrochloride
The commonly used dosage form of 3-Hydroxytyramine hydrochloride is injection. It is a dopamine receptor agonist. It is suitable for shock syndrome caused by myocardial infarction, trauma, endotoxin sepsis, heart surgery, renal failure, congestive heart failure, etc. If the shock cannot be corrected after volume replacement, especially oliguria and shock with normal or low peripheral vascular resistance. Since this product increases cardiac output, it is also used for cardiac insufficiency where digitalis and diuretics are ineffective [11].
Adult dosage: intravenous injection. At the beginning, 1-5μg/kg body weight per minute was increased at a rate of 1-4μg/kg per minute within 10 minutes to achieve maximum efficacy [12].
Chronic intractable heart failure, at the beginning of intravenous drip, 0.5 to 2μg/kg weight per minute gradually increase. Most patients are given 1-3μg/kg per minute to take effect.
For patients with occlusie vascular disease, intravenous drip was started at 1μg/kg per minute and gradually increased to 5-10μg/kg per minute until 20μg/kg per minute to achieve the most satisfactory effect.
In critical cases, first drip 5μg/kg per minute, and then increase to 20-50μg/kg per minute by 5-10μg/kg to achieve satisfactory effect. Or add 20mg of this product into 5% glucose injection 200-300ml intravenous drop, at the beginning of 75-100μg/min drop, later according to the blood pressure, can be speeded up and increase the concentration, but the maximum dose does not exceed 500μg /min.
Pharmacological effects
3-Hydroxytyramine hydrochloride excites adrenergic receptors of sympathetic nervous system and dopamine receptors located in kidney, mesentery, coronary artery and cerebral artery in a dose-dependent manner [13].
At a small dose (0.5-2 ug/kg per minute according to body weight), 3-Hydroxytyramine hydrochloride mainly acts on dopamine receptors, which dilates renal and mesenteric blood vessels, increases renal blood flow and glomerular filtration rate, and increases urine volume and sodium excretion [14].
Small to medium dose per minute (2-10 ug/kg by weight), it can directly excite beta 1 receptor and indirect prompted norepinephrine from storage area to release, have positive stress effects on myocardial, increase the amount of myocardial contraction and cardiac, eventually increase the cardiac output, higher systolic blood pressure, diastolic pressure moderately elevated or have no change.
When the large dose (per minute according to the weight of more than 10 ug/kg), it can excite the α receptor, resulting in increased peripheral vascular resistance, renal vascular contraction, renal blood flow and urine volume decreased instead. Systolic and diastolic blood pressure increased due to increased cardiac output and peripheral vascular resistance [15].
By increasing blood flow to the kidneys and mesentery, it prevents the malignant development of shock due to ischemia of these organs. In the same condition of increasing myocardial contractility, arrhythmia and increasing myocardial oxygen consumption are weak. In summary, dopamine is particularly useful in shock patients with reduced myocardial contractility and reduced urine volume, with blood volume already replenished.
Drug interaction
When combined with sodium nitroprusside, isoproterenol and dobutamine, attention should be paid to the change of cardiac output. The reaction is different than when using this product alone.
In conjunction with beta-blockers, this product antagonizes the effect of dopamine on cardiac beta-1 receptors [16].
Combined with general anesthetics (especially cyclopropane or halogenated hydrocarbons), the latter can make the myocardium abnormally sensitive to dopamine and cause ventricular arrhythmias.
High doses of dopamine are equated with alpha-blockers such as phenbenzamine, phenolamine, and Tolazoline, whose vasodilatory effects are antagonized by peripheral vascular constriction.
Similar with nitrate, it can weaken the anti-angina pectoris and dopamine pressure boosting effect of nitrate [17].
Adverse reactions
Common symptoms include chest pain, dyspnea, palpitations, arrhythmias (especially in large doses), and general weakness [18]. Slow heartbeat, headache, nausea and vomiting are rare. Long-term application of high dose or low dose for peripheral vascular disease, the reaction of hand and foot pain or hand and foot cooling; Prolonged peripheral vascular contraction may lead to local necrosis or gangrene. Elevated blood pressure may occur in excess, at which point the drug should be discontinued and alpha-blockers given if necessary.
Ecological data
According to Water Hazard Class 1 (German Regulation) (self-assessment by list), the substance is slightly hazardous to water [19].
Do not allow undiluted or large quantities of products to come into contact with groundwater, waterways or sewage systems.
Do not release materials into the environment unless anarchy permits
References
[1] J. Baird, J. Lewis, Effects of drugs on noradrenaline and 3-hydroxytyramine (dopamine) levels and on the noradrenaline to dopamine ratio in the rat brain, Biochemical Pharmacology 12(6) (1963) 579-581.
[2] W.M. Bennett, E. Keeffe, C. Melnyk, D. Mahler, J. Rösch, G.A. Porter, Response to dopamine hydrochloride in the hepatorenal syndrome, Archives of internal medicine 135(7) (1975) 964-971.
[3] R. Bergin, D. Carlström, The structure of the catecholamines. II. The crystal structure of dopamine hydrochloride, Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 24(11) (1968) 1506-1510.
[4] F. Binns, R. Chapman, N. Robson, G. Swan, A. Waggott, Studies related to the chemistry of melanins. Part VIII. The pyrrolecarboxylic acids formed by oxidation or hydrolysis of melanins derived from 3, 4-dihydroxyphenethylamine or (±)-3, 4-dihydroxyphenylalanine, Journal of the Chemical Society C: Organic (8) (1970) 1128-1134.
[5] T.M. Bustard, R. Egan, The conformation of dopamine hydrochloride, Tetrahedron 27(19) (1971) 4457-4469.
[6] S. Bygdeman, Noradrenaline, 3-hydroxytyramine and related compounds in Solanum tuberosum, Ark. Kemi 16 (1960) 247-53.
[7] S.K. Callear, A. Johnston, S.E. McLain, S. Imberti, Conformation and interactions of dopamine hydrochloride in solution, The Journal of Chemical Physics 142(1) (2015) 01B601_1.
[8] A. CARLSSON, B. WALDECK, A fluorimetric method for the determination of dopamine (3‐hydroxytyramine.), Acta physiologica scandinavica 44(3‐4) (1958) 293-298.
[9] J.E. Carter, J.H. Johnson, D.M. Baaske, Dopamine hydrochloride, Analytical profiles of drug substances, Elsevier1982, pp. 257-272.
[10] D.R. Dreyer, D.J. Miller, B.D. Freeman, D.R. Paul, C.W. Bielawski, Elucidating the structure of poly (dopamine), Langmuir 28(15) (2012) 6428-6435.
[11] M. Goldstein, A. Friedhoff, C. Simmons, Metabolic pathways of 3-hydroxytyramine, Biochimica et Biophysica Acta 33(2) (1959) 572-574.
[12] M. Goldstein, A. Friedhoff, C. Simmons, N. Prochoroff, The metabolism of 3-hydroxytyramine-1-C14 in brain tissue homogenates, Experientia 15(7) (1959) 254-256.
[13] Z.E. Koç, M.O. Aladag, A. Uysal, Synthesis of novel dopamine derived multidirectional ligands from cyanuric chloride: structural and antimicrobial studies, EXCLI journal 12 (2013) 396.
[14] Q. Li, J. Li, Z. Yang, Study of the sensitization of tetradecyl benzyl dimethyl ammonium chloride for spectrophotometric determination of dopamine hydrochloride using sodium 1, 2-naphthoquinone-4-sulfonate as the chemical derivative chromogenic reagent, Analytica Chimica Acta 583(1) (2007) 147-152.
[15] E. McGeer, P. McGeer, H. McLennan, The inhibitory action of 3‐hydroxytyramine, gamma‐aminobutyric acid (GABA) and some other compounds towards the crayfish stretch receptor neuron, Journal of Neurochemistry 8(1) (1961) 36-49.
[16] O. Mostovaya, P. Padnya, A. Vavilova, D. Shurpik, B. Khairutdinov, T. Mukhametzyanov, A. Khannanov, M. Kutyreva, I. Stoikov, Tetracarboxylic acids on a thiacalixarene scaffold: Synthesis and binding of dopamine hydrochloride, New Journal of Chemistry 42(1) (2018) 177-183.
[17] E. Sanner, Tubular Excretion of Dopamine (3‐hydroxytyramine) by the Chicken Kidney, Acta Pharmacologica et Toxicologica 20(4) (1964) 375-384.
[18] S. Senoh, B. Witkop, C. Creveling, S. Udenfriend, 2, 4, 5-trihydroxyphenethylamine, a new metabolite of 3, 4-dihydroxyphenethylamine, Journal of the American Chemical Society 81(7) (1959) 1768-1769.
[19] J. Shashikumara, B.K. Swamy, K. Chetankumar, Sensitive and selective sensor for 3, 4-dihydroxyphenethylamine and uric acid at poly (Orange CD) modified carbon paste electrode, Chemical Data Collections 32 (2021) 100661.
- Related articles
- Related Qustion
- Side effects of Dopamine hydrochloride Nov 15, 2021
Dopamine hydrochloride, a sympathomimetic amine vasopressor, is the naturally occurring immediate precursor of norepinephrine. Dopamine hydrochloride is a white to off-white crystalline powder, which may have a slight odor of hydrochloric a
Sodium sulfite is a white, water-soluble, crystalline solid with a sulfurous, salty taste. It decomposes when heated. It is generally available f in powder, crystalline, and tablet forms.....
Jul 12,2022Inorganic chemistryThe physiological substance and precursor of the heme synthesis 5-aminolevulinic acid (ALA) is a promising prodrug for photodiagnosis and photodynamic therapy of epithelial tumors.....
Jul 12,2022APIDopamine hydrochloride
62-31-7You may like
Dopamine hydrochloride manufacturers
- 3-Hydroxytyramine hydrochloride
-

- $20.00 / 1kg
- 2024-04-29
- CAS:62-31-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 300tons
- 3-Hydroxytyramine hydrochloride
-

- $6.00 / 1KG
- 2024-04-24
- CAS:62-31-7
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 2000KG/Month
- Dopamine hydrochloride
-

- $0.00 / 1kg
- 2024-04-22
- CAS:62-31-7
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 1000 kg