Propylparaben: A Comprehensive Overview for Chemical Professionals
Apr 28,2024
Introduction
Propylparaben, scientifically termed propyl p-hydroxybenzoate, belongs to the paraben family of chemicals, which are esters derived from para-hydroxybenzoic acid and different alcohols. This compound is particularly noteworthy due to its widespread adoption as a preservative across multiple key industries, including cosmetics, pharmaceuticals, and food processing. Its efficacy in preventing microbial growth makes it an invaluable component in the formulation of products that require extended shelf life and enhanced safety. The broad application of propylparaben has drawn significant attention from both industry professionals and researchers, leading to ongoing studies about its safety and environmental impact. This scrutiny is partly due to rising consumer awareness and regulatory concerns about the potential health effects associated with long-term exposure to parabens.

Figure 1 Characteristics of Propylparaben
Synthesis
The synthesis of propylparaben typically involves the esterification of p-hydroxybenzoic acid with propanol. This reaction is often catalyzed by an acid such as sulfuric acid, which facilitates the removal of water formed during the process. The synthesis can be conducted under reflux to ensure that the reaction temperature is maintained consistently. Advances in synthesis techniques have also explored enzymatic methods, which offer a greener alternative by minimizing the use of harsh chemicals and reducing by-products.
Main Components
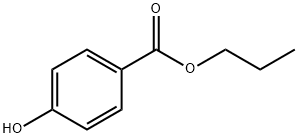
Propylparaben is characterized by its chemical structure consisting of a benzene ring substituted with a hydroxyl group (OH) and an ester group linking to a propyl chain. This structure is pivotal in its ability to inhibit the growth of microbes by interfering with their nutrient intake process. The presence of the hydroxyl group enhances the hydrophilicity of the compound, while the propyl group balances its lipophilicity, making it effective in a variety of mediums.
Uses
Propylparaben, a widely utilized preservative known for its effective antimicrobial properties, plays a critical role in extending the shelf life of products by inhibiting the growth of fungi, bacteria, and yeast. Its application spans across various industries, prominently in cosmetics where it helps preserve the freshness and integrity of products. In the pharmaceutical field, propylparaben is a key ingredient in numerous formulations, acting as a safeguard against contamination, thus ensuring the safety and efficacy of medicinal products. The use of propylparaben in food preservation is also notable, although it is carefully regulated to address health concerns linked to its consumption. These regulations are in place to prevent potential health risks, reflecting the need for careful management of its use in consumer products.
Precautions
While propylparaben is generally recognized as safe for use in various applications, several precautions must be considered. Recent studies have raised concerns regarding the potential endocrine-disrupting effects of parabens, including propylparaben, which may mimic estrogen and interfere with hormone function in humans. Regulatory agencies such as the FDA and the European Chemicals Agency (ECHA) monitor the use of such chemicals and set limits on their concentration in consumer products.
Regulatory scrutiny has also led to the development of alternative preservation strategies that might reduce or eliminate the need for parabens. This is in response to consumer demand for more natural products and concerns about long-term exposure to synthetic preservatives. Chemical professionals are encouraged to stay updated with the latest research and regulatory changes to ensure compliance and optimal use of propylparaben in their formulations.
Conclusion
Propylparaben remains a vital component in various industries due to its effective preservative properties. However, the ongoing research into its safety profile and regulatory adjustments poses challenges for professionals in the chemical sector. It is imperative that these professionals not only understand the synthesis, properties, and applications of propylparaben but also keep abreast of the evolving landscape regarding its use and the development of safer, more sustainable alternatives. By doing so, they can ensure that their practices align with both industry standards and public health guidelines, thereby upholding the integrity of their products and the safety of consumers.
References
[1]Andersen F A. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products[J]. Int J Toxicol, 2008, 27(Suppl 4): 1-82.
[2]Martín J M P, Peropadre A, Herrero ó, et al. Oxidative DNA damage contributes to the toxic activity of propylparaben in mammalian cells[J]. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2010, 702(1): 86-91.
- Related articles
- Related Qustion
- Propylparaben: A Common Antimicrobial and Preservative Chemical Found in Personal Care Products and Food Dec 20, 2023
Propylparaben, a paraben compound, is widely used for its antimicrobial and preservative properties in various consumer products. The majority of it is excreted as non-specific metabolites.
- Propylparaben in Skincare Nov 29, 2022
The passage talks about how Propylparaben works in skincare.
Propylparaben
94-13-3You may like
- Propylparaben
-

- $18.00 / 10kg
- 2024-05-10
- CAS:94-13-3
- Min. Order: 1kg
- Purity: 99.9
- Supply Ability: 5000
- Propylparaben
-

- $10.00 / 1kg
- 2024-05-10
- CAS:94-13-3
- Min. Order: 1kg
- Purity: 99.5
- Supply Ability: 10 ton per month
- Propylparaben
-

- $10.00 / 1kg
- 2024-05-10
- CAS:94-13-3
- Min. Order: 1kg
- Purity: 99.5
- Supply Ability: 10 ton per month