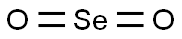Selenium Dioxide: Applications in Synthesis of Nanocrystals and Removal from Flue Gas
Apr 30,2024
General Description
Selenium dioxide, a white crystalline solid with oxidizing properties, is utilized in various industrial applications. It serves as a crucial precursor in the synthesis of high-quality ZnSe nanocrystals, offering a green alternative to traditional methods. The compound is also effective in the removal of sulfur dioxide and trace selenium dioxide from flue gas, with calcium oxide demonstrating significant adsorption capabilities. Despite the potential toxicity of selenium dioxide, its unique properties make it a valuable component in technological advancements, showcasing versatility in tailored nanocrystal synthesis and environmental remediation processes.

Figure 1. Selenium dioxide
Overview
Selenium dioxide, chemically represented as SeO2, is a compound of selenium and oxygen. It exists as a white crystalline solid and exhibits oxidizing properties. Selenium dioxide finds various applications in industrial processes, including as a reagent in organic synthesis and as a catalyst in certain chemical reactions. Its unique properties make it a valuable component in several technological advancements. However, it should be handled with care due to its potential toxicity. 1
Applications in Synthesis of Nanocrystals
Selenium dioxide serves as a versatile and cost-effective precursor in the synthesis of nanocrystals, particularly evident in the production of high-quality zinc blende (cubic) ZnSe nanocrystals. This innovative approach offers a "green" alternative to traditional methods by circumventing the need for expensive and toxic solvents like trioctylphosphine (TOP) or tributylphosphine (TBP). Instead, Selenium dioxide is dispersed in 1-octadecene (ODE), functioning as a chalcogen precursor. The synthesis process is meticulously controlled to achieve desired size and shape variations in the ZnSe nanocrystals. Factors such as temperature and surface ligand play pivotal roles in influencing nucleation, reaction kinetics, and resultant morphology. Characterization techniques including absorption spectroscopy, fluorescence spectroscopy, powder X-ray diffraction (XRD), and transmission electron microscopy (TEM) are employed to assess the quality and properties of the synthesized nanocrystals. Remarkably, the size-dependent photoluminescence (PL) range of the ZnSe nanocrystals falls between 390 and 450 nm, with the PL full width at half-maximum (FWHM) tightly controlled within a narrow range of 14 to 18 nm. Additionally, the PL quantum yields reach remarkable levels of up to 40% at room temperature, indicative of their high efficiency in light emission. Furthermore, this novel selenium precursor facilitates the formation of tetrapod-shaped ZnSe nanocrystals through a convenient one-pot synthesis approach when combined with zinc acetylacetonate as the zinc precursor. This underscores the versatility and applicability of Selenium dioxide in the tailored synthesis of nanocrystals with diverse morphologies and optical properties. 2
Removal from Flue Gas
The removal of selenium dioxide from flue gas is a significant concern due to its harmful effects on the environment and human health. In the context of coal combustion, sulfur dioxide (SO2) and trace selenium dioxide are pollutants emitted into the atmosphere. This study focuses on the simultaneous removal of sulfur and trace selenium dioxide by calcium oxide (CaO) adsorption at medium temperatures, with particular emphasis on the mass transfer effects of sulfate product layers on trace elements. Experimental findings demonstrate that the presence of a product layer introduces additional mass transfer resistance to the sorbent-gas reaction process. However, as the concentration of SO2 decreases, the extent of CaO adsorption ability loss due to this factor diminishes. At trace gas concentrations, the loss of CaO adsorption ability becomes negligible. Furthermore, experiments investigating the adsorption of trace selenium dioxide gas by CaO indicate that the sulfate product layer, regardless of its thickness, does not significantly affect CaO's ability to adsorb trace selenium dioxide gas. Overall, these findings highlight the efficacy of calcium oxide in simultaneously removing sulfur dioxide and trace selenium dioxide from flue gas, with the understanding that the presence of a sulfate product layer may introduce some mass transfer resistance, but its impact diminishes at lower SO2 concentrations. Additionally, the presence of a sulfate product layer does not hinder CaO's ability to adsorb trace selenium dioxide gas. 3
Reference
1. Selenium dioxide. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 24007.
2. Shen H, Niu JZ, Wang H, Li X, Li LS, Chen X. Size- and shape-controlled synthesis of ZnSe nanocrystals using SeO2 as selenium precursor. Dalton Trans. 2010; 39(47): 11432-11438.
3. Li Y, Tong H, Zhuo Y, Chen C, Xu X. Simultaneous removal of SO2 and trace SeO2 from flue gas: effect of product layer on mass transfer. Environ Sci Technol. 2006;40(13):4306-4311.
- Related articles
- Related Qustion
- Selenium dioxide: properties, applications and safety Dec 15, 2023
Selenium dioxide is a versatile, oxidizing compound used in various industries, but it requires careful handling due to its toxicity and potential health risks.
- Selenium Dioxide Oxidation Jan 28, 2023
Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones.
- Application and Pharmacology of Selenium dioxide Jun 29, 2022
Selenium dioxide appears as a white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C.
Sodium thiocyanate is a significant chemical in the industrial landscape, marked by its versatile applications and critical role in synthesis processes.....
Apr 30,2024APID-Mannose prevents urinary tract infections by inhibiting bacterial adhesion. It's effective with minimal adverse events, mainly mild diarrhea, making it safer than nitrofurantoin.....
Apr 30,2024APISelenium dioxide
7446-08-4You may like
Selenium dioxide manufacturers
- Selenium dioxide
-

- $0.00 / 25KG
- 2023-09-23
- CAS:7446-08-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Selenium(IV) oxide
-

- $0.00 / 1kg
- 2023-09-06
- CAS:7446-08-4
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 100tons
- Selenium dioxide
-

- $0.00 / 1KG
- 2023-09-06
- CAS:7446-08-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg