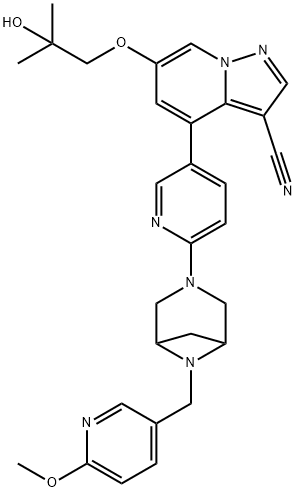
Selpercatinib: Synthesis and Description
Jan 5,2024
Synthesis of Selpercatinib
Selpercatinib (LOXO-292) is synthesised using sulfonyl chloride as a raw material by chemical reaction. The specific synthesis steps are as follows:

The sulfonyl chloride starting material 329 was reacted with tert-butyl hydroxycarbamate (330) in base followed by treatment with TFA to obtain Osulfonyl hydroxylamine 332. This system served as an electrophilic source of nitrogen in a reaction with pyridine 333 to furnish the N-aminopyridyl salt 334, which was further reacted with chloroacrylonitrile (335) to establish the pyrazolopyridine core 336. Removal of the methyl ether using dodecanethiol 337 under basic conditions in warm DMF was followed by the conversion of the resulting phenol 338 to the triflate 339. Cross-coupling of 339 and boronate 340 to give 341 occurred exclusively at the triflate position, sparing the bromide under Suzuki conditions at lower temperatures than usual for this reaction. Subjection of 341 to epoxide 342 under palladiumcatalyzed conditions established the ethereal side chain, and this was followed by nucleophilic aromatic substitution to install the azabicyclic appendage 344 in 86% yield, which resulted in formation of selpercatinib.
Description of Selpercatinib
Selpercatinib is a highly selective receptor tyrosine kinase RET inhibitor. It is used for the treatment of various solid tumours containing RET alterations. On May 8, 2020, selpercatinib was approved by the USFDA for the treatment of RET fusion-positive nonsmall-cell lung cancer, RET fusion-positive thyroid cancer, and RET mutant medullary thyroid cancer. The approval was granted on the basis of the clinically important effects on the overall response rate (ORR) of 64% in prior platinum treated patients of RET fusion-positive NSCLC, 69% in previously treated patients of advanced or metastatic RET mutant medullary thyroid cancer, and 79% in previously treated RET fusion-positive thyroid cancer patients.
- Related articles
- Related Qustion
- LOXO-292: A Promising RET Inhibitor with Strong Anti-Tumor Effects and Manageable Adverse Events Mar 4, 2024
LOXO-292 inhibits RET-pathway, demonstrating anti-tumor effects and dose-proportional pharmacokinetics. Common adverse events occurred, with serious reactions in 33% of patients.
- LOXO-292: Bioactivity and Chemicaland toxicological studies Dec 20, 2022
LOXO-292 is a selective and potent RET inhibitor,it is a novel broad-spectrum anticancer?drug with the targeted?function for RET protooncogene.There are three synthetic routes to selp
Triclabendazole, also known as CGA 89317 or CGP 23030, is an orally bioavailable anthelmintic against infections caused by Fasciola species.....
Jan 5,2024APISelumetinib Sulfate was prepared by a two-step chemical reaction by first synthesising Selumitinib Benzimidazole intermediates using 2,3,4-trifluorobenzoic acid as starting material.....
Jan 5,2024InhibitorsLOXO-292
2152628-33-4You may like
- LOXO-292
-

- $6.00 / 1kg
- 2024-06-03
- CAS:2152628-33-4
- Min. Order: 1kg
- Purity: More than 99%
- Supply Ability: 2000KG/Month
- Selpercatinib
-

- $80.00 / 1kg
- 2024-06-03
- CAS:2152628-33-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- LOXO-292
-

- $50.00 / 1KG
- 2024-06-03
- CAS:2152628-33-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500