Synthesis and Application of GW-501516
Oct 10,2022
Generally speaking
GW 501516 is shown to be the most potent and selective PPARα agonists known with an EC50 of 1.1 nM against PPARα and 1000-fold selectivity over the other human subtypes, PPARα and-γ. GW 501516 exerts anti-inflammatory effects in mouse cultured proximal tubular (mProx) cells. GW 501516 inhibits palmitate- and TNFα-induced increases in MCP-1 mRNA expression in a dose-dependent manner.
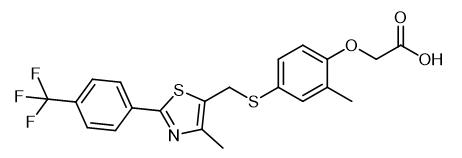
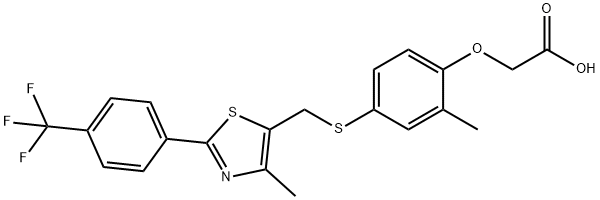
Fig. 1 The structure of GW-501516.
Synthetic routes
Fig. 2 The synthetic step 1 of GW-501516.
Add Cs2CO3 (3.25 g, 10.0 mmol), followed by the chloromethylthiazole (2.60 g) to a stirred solution of 4-mercapto-2-methylphenol (1.40 g) in CH3CN (80 mL). Stir the reaction mixture at room temperature for 4 hours. Add Cs2CO3 (4.89 g, 15.0 mmol), followed by methyl bromoacetate (1.23 mL, 13.0 mmol) to the mixture. Stir the reaction mixture at room temperature for another 5 hours. Pour the mixture into water. Extract the mixture with EtOAc (3 x 100 mL). Combine the organic layers. Wash the organic layers with brine. Dry the organic layers (Na2SO4). Concentrate the organic layers. Purify the residue by column chromatography on silica gel with hexane/ ethyl acetate (5:1). 1H NMR (CDCl3) δ7.97 (d, 2H, J= 8.4 Hz), 7.65 (d, 2H, J= 8.4 Hz), 7.21 (d, 1H, J= 2.4 Hz), 7.13 (dd, 1H, J= 8.4, 2.4 Hz), 6.58 (d, 1H, J= 8.4 Hz), 4.63 (s, 2H), 4.11 (s, 2H), 3.78 (s, 3H), 2.24 (s, 3H), 2.20 (s, 3H). 13C NMR (CDCl3) δ169.2, 163.0, 156.3, 151.3, 136.8, 136.1, 132.1, 131.2 (q, J= 32 Hz), 130.6, 128.4, 126.3, 125.8 (q, J= 4 Hz), 125.3, 122.1, 111.4, 65.4, 52.2, 32.4, 16.1, 14.8. 19F NMR (CDCl3) δ115.5 (s) [1].
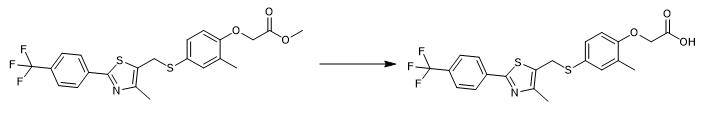
Fig. 3 The synthetic step 2 of GW-501516.
Add 6.0 mL (12.0 mmol) of 2.0 M LiOH slowly to a stirred solution of methyl [2-methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)-phenyl]thiazol-5- yl]methyl]sulfanyl]phenoxy]acetate (4.5 g) in 150 mL of THF and 100 mL of H2O at 0 °C. Stir the reaction mixture at 0 °C. Monitor the completion of the reaction by TLC (about 1 hour). Dilute the reaction mixture with water (100 mL). Acidify the reaction mixture with 0.5 M NaHSO4 (25 mL). Extract the reaction mixture with a mixed solvent of EtOAc and THF (3:1, 4 x 150 mL). Combine the organic layers. Dry the organic layers (Na2SO4). Concentrate the organic layers. Purify the residue by column chromatography on silica gel with CH2Cl2/MeOH (10:1). 1H NMR (DMSO-d6) δ8.02 (d, 2H, J= 8.4 Hz), 7.79 (d, 2H, J= 8.4 Hz), 7.20 (s, 1H), 7.13 (d, 1H, J= 8.1 Hz), 6.70 (d, 1H, J= 8.1 Hz), 4.46 (s, 2H), 4.31 (s, 2H), 2.20 (s, 3H), 2.13 (s, 3H). 13C NMR (DMSO-d6) δ171.3, 161.6, 156.4, 150.9, 136.4, 134.1, 131.6, 130.7, 129.5 (q, J= 32 Hz), 126.7, 126.2, 126.0 (q, J= 4 Hz), 123.4, 111.9, 66.2, 30.6, 15.9, 14.6. 19F NMR (DMSO-d6) δ115.5 (s) [2].
Application
As the PPAR-beta agonist
Brain inflammation plays a central role in numerous brain pathologies, including multiple sclerosis (MS). Microglial cells and astrocytes are the effector cells of neuroinflammation. They can be activated also by agents such as interferon-gamma (IFN-gamma) and lipopolysaccharide (LPS). Peroxisome proliferator-associated receptor (PPAR) pathways are involved in the control of the inflammatory processes, and PPAR-beta seems to play an important role in the regulation of central inflammation. In addition, PPAR-gamma agonists were shown to have trophic effects on oligodendrocytes in vitro, and to confer partial protection in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. In the present work, a three-dimensional brain cell culture system was used as in vitro model to study antibody-induced demyelination and inflammatory responses. GW 501516, a specific PPAR-gamma agonist, was examined for its capacity to protect from antibody-mediated demyelination and to prevent inflammatory responses induced by IFN-gamma and LPS. Aggregating brain cells cultures were prepared from embryonal rat brain, and used to study the inflammatory responses triggered by IFN-gamma and LPS and by antibody-mediated demyelination induced by antibodies directed against myelin-oligodendrocyte glycoprotein (MOG). The effects of GW 501516 on cellular responses were characterized by the quantification of the mRNA expression of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), inducible NO synthase (i-NOS), PPAR-beta, PPAR-gamma, glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), and high molecular weight neurofilament protein (NF-H). GFAP expression was also examined by immunocytochemistry, and microglial cells were visualized by isolectin B4 (IB4) and ED1 labeling. GW 501516 decreased the IFN-gamma-induced up-regulation of TNF-alpha and iNOS in accord with the proposed anti-inflammatory effects of this PPAR-beta agonist. However, it increased IL-6 m-RNA expression. In demyelinating cultures, reactivity of both microglial cells and astrocytes was observed, while the expression of the inflammatory cytokines and iNOS remained unaffected. Furthermore, GW 501516 did not protect against the demyelination-induced changes in gene expression. Although GW 501516 showed anti-inflammatory activity, it did not protect against antibody-mediated demyelination. This suggests that the protective effects of PPAR-beta agonists observed in vivo can be attributed to their anti-inflammatory properties rather than to a direct protective or trophic effect on oligodendrocytes [2].
It has been reported that treatment of cultured human skeletal muscle myotubes with the peroxisome proliferator-activated receptor-delta (PPAR delta) activator GW-501516 directly stimulates glucose transport and enhances insulin action. Cultured myotubes are minimally responsive to insulin stimulation of glucose transport and are not a good model for studying skeletal muscle glucose transport. The purpose of this study was to evaluate the effect of GW-501516 on glucose transport to determine whether the findings on cultured myotubes have relevance to skeletal muscle. Rat epitrochlearis and soleus muscles were treated for 6 h with 10, 100, or 500 nM GW-501516, followed by measurement of 2-deoxyglucose uptake. GW-501516 had no effect on glucose uptake. There was no effect on insulin sensitivity or responsiveness. Also, in contrast to findings on myotubes, treatment of muscles with GW-501516 did not result in increased phosphorylation or increased expression of AMP-activated protein kinase ( AMPK) or p38 mitogen-activated protein kinase ( MAPK). Treatment of epitrochlearis muscles with GW-501516 for 24 h induced a threefold increase in uncoupling protein-3 mRNA, providing evidence that the GW-501516 compound that we used gets into and is active in skeletal muscle. In conclusion, our results show that, in contrast to myotubes in culture, skeletal muscle does not respond to GW-501516 with 1) an increase in AMPK or p38 MAPK phosphorylation or expression or 2) direct stimulation of glucose transport or enhanced insulin action [3].
Renin-angiotensin system (RAS) regulates adipogenic response with adipocyte hypertrophy by increasing oxidative stress. Rec
- Related articles
- Related Qustion
- GW-501516: Benefits, Clinical Applications and Dosage Mar 11, 2024
GW-501516 enhances performance and fat loss but is limited by cancer risks and lacks clinical authorization, necessitating careful risk-benefit evaluation.
- The side effects of GW-501516 Sep 27, 2023
GW-501516 is a peroxisome proliferator-activated receptor (PPAR) agonists. It may cause several side effects.
GW-501516
317318-70-0You may like
- GW-501516Cardarine
-

- $40.00 / 1Box
- 2024-04-29
- CAS:317318-70-0
- Min. Order: 1Box
- Purity: 99.99%
- Supply Ability: 10000000000
- GW501516(Cardarine)
-

- $9.90 / 1g
- 2024-04-29
- CAS:317318-70-0
- Min. Order: 100g
- Purity: 99.99%
- Supply Ability: 100kg
- GW-501516
-

- $0.00 / 1G
- 2024-04-29
- CAS:317318-70-0
- Min. Order: 1G
- Purity: 99%
- Supply Ability: 20