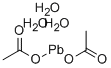
Synthesis, Toxicology and Application of Lead acetate trihydrate
Nov 14,2022
General description
Lead acetate trihydrate is colorless crystal, white particle or powder, will deliquescence. It is easily soluble in water and has a sweet taste. Lead acetate trihydrate is used to make all kinds of lead salt, pigment, dye, lead plating, polyester catalyst, waterproof paint, desiccant, insecticide and medicine.
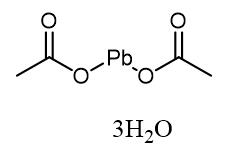
Fig. 1 The structure of Lead acetate trihydrate.
Preparation
Lead oxide is obtained by reacting with acetic acid. The lead oxide was dissolved in 80% hot acetic acid until it was bleached and filtered. After adding a small amount of acetic acid to the filtrate, it was evaporated to a relative density of 1.40. After cooling, filtration and drying, lead acetate was obtained. The purity of lead acetate in industrial products can be more than 98%. After purification, 1% acetic acid solution can be recrystallized, or lead acetate trihydrate is dissolved in water through hydrogen sulfide, lead sulfide and other impurities together precipitation, after filtration, fluorescent gallium reagent (Co, Al, Cu complexing agent) is added to the filtrate, a small amount of activated carbon, and then EDTA sodium salt treatment, very pure reagent grade products can be obtained.
Electrochemical characterization
The effect of lead acetate trihydrate on platinum black coating has been examined for the electrolyte five of or including lead acetate trihydrate using cyclic potential-current curves and a multi-pulse current measurement. The current-potential curves measured with a cyclic sweep of a current density in the electrolyte including lead acetate trihydrate show that no peak assigned to the reduction of Pt (IV) to Pt (II) in the process of electrode reactions is observed. The kinetic constant, the exchange current density and the double-layer capacitance, which can be determined from the measured potential-time curves, exhibit significantly different values for H2PtCl6 electrolytes free of or containing lead acetate tribydrate. In particular, the exchange current density for the electrolyte including lead acetate trihydrate is much larger than that for the electrolyte free of lead acetate trihydrate. These experimental results indicate that lead acetate trihydrate significantly enhances the electrode reactions in platinum black coating by mainly lowering a height of the energy barrier for the reduction of Pt (IV) to Pt (0) and by suppressing the reduction of Pt (IV) to Pt (II) [1].
Toxicity experiment
SPF male Wistar rats were exposed for four months to lead acetate trihydrate present in drinking water (100 mg/l) and subsequently infested with 1000 +/- 100 infective A. suum eggs. Metabolic activity of phagocytes and proliferative activity of T-lymphocytes were investigated on Day 4 and 8 after A. suum eggs infestation (Day 130 and 134 of lead acetate trihydrate exposition). The results demonstrated that treatment with lead acetate trihydrate led to increased susceptibility to infestation, manifested by increased average number of A. suum larvae in the lungs of exposed rats compared to unexposed ones. Moreover, migration of A. suum larvae on Day 8 was associated with significant increase in index of metabolic activity of phagocytes in unexposed rats in comparison with controls. In contrast, in rats exposed to the lead and infested by A. suum eggs a non-significant increase in the studied immunological parameters was recorded. Significant differences in immunological parameters were observed between unexposed, infested and infested and exposed groups of rats. In the unexposed group of animals Ascaris suum infestation caused a significant increase in the index of metabolic activity of phagocytes and stimulation index of lymphocytes in comparison with lead exposed rats [2].
Lead increasingly contributes to pollution of the environment and may play a role in the development of adverse effects in the human and animal body. Data concerning its mutagenic, clastogenic, and carcinogenic properties have been conflicting. In this study, we evaluated the frequency of micronuclei in bone marrow erythrocytes of rats treated with lead acetate trihydrate. Outbred Wistar rats were exposed to a daily dose of 100 mg/L drinking water for 125 days. The mean value of the total number of micronuclei observed in polychromatic erythrocytes of female rats was significantly higher than that found in the control group (13.375 +/- 2.722 against 9.625 +/- 3.204 micronuclei/1000 cells; P = 0.024 in ANOVA). In exposed female animals, no significant reduction of the ratio of polychromatic to normochromatic erythrocytes was observed (0.990 +/- 0.228 against 1.208 +/- 0.195; P = 0.060 in ANOVA). The effects of lead acetate trihydrate in male rats are both cytotoxic and genotoxic because of a decrease in ratio of polychromatic to normochromatic erythrocytes (0.715 +/- 0.431 against 1.343 +/- 0.306; P = 0.023, ANOVA followed by Tukey test) and an increase in frequency of micronucleated polychromatic erythrocytes (24.167 +/- 7.859 against 4.0 +/- 4.528 micronuclei/1000 cells; P <= 0.001, ANOVA followed by Tukey test), respectively [3].
Application
Synthesis of novel ultrafine lead oxide
A novel green recycling process is investigated to prepare lead acetate trihydrate precursors and novel ultrafine lead oxide from spent lead acid battery pastes. The route contains the following four processes. (1) The spent lead pastes are desulphurized by (NH4)(2)CO3.(2)The desulphurized pastes are converted into lead acetate solution by leaching with acetic acid solution and H2O2; (3) The Pb(CH3COO)(2)center dot 3H(2)O precursor is crystallized and purified from the lead acetate solution with the addition of glacial acetic acid; (4) The novel ultrafine lead oxide is prepared by the calcination of lead acetate trihydrate precursor in N-2 or air at 320 -400 degrees C. Both the lead acetate trihydrate and lead oxide products are characterized by TG-DTA, XRD, and SEM techniques. The calcination products are mainly alpha-PbO, beta-PbO, and a small amount of metallic Pb. The particle size of the calcination products in air is significantly larger than that in N-2. Cyclic voltammetry measurements of the novel ultrafine lead oxide products show good reversibility and cycle stability. The assembled batteries using the lead oxide products as cathode active materials show a good cyclic stability in 80 charge/discharge cycles with the depth of discharge (DOD) of 100% [4].
As cold sinter-assisted
Ceramics such as lead zirconate titanate (PZT) tend to dissolve incongruently, and thus pose a challenge in the cold sintering process. Moist lead nitrate has previously been shown to enable a cold sinter-assisted densification of PZT by a viscous phase sintering mechanism. In this paper, lead acetate trihydrate is demonstrated to lower the required temperature of the cold sintering step to 200 degrees C. This densification process was described as a two-step process: cold sintering of PZT with lead acetate trihydrate and post-annealing the as-cold sintered PZT ceramics. Unlike in the case of lead nitrate, PZT densification with lead acetate trihydrate occurs by a liquid phase assisted sintering mechanism, leading to an as-cold sintered relative density of 84% at 200 degrees C. After performing a post-anneal step at 900 degrees C, >97% relative densities were achieved in samples that were cold sintered with lead acetate trihydrate. This step not only densified PZT but also refined the grain boundaries. In the post-annealed samples, the room-temperature relative permittivity at 100 Hz was similar to 1600, slightly higher than that reported in samples that used lead nitrate as a sintering aid; the loss tan
- Related articles
- Related Qustion
Oseltamivir acid is an intermediate in the preparation of oseltamivir.....
Nov 14,2022Pharmaceutical intermediatesLead acetate trihydrate
6080-56-4You may like
Lead acetate trihydrate manufacturers
- Lead acetate trihydrate
-

- $10.00 / 1kg
- 2024-04-29
- CAS:6080-56-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 300tons
- Lead acetate trihydrate
-

- $10.00/ kg
- 2024-04-25
- CAS:6080-56-4
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 10000ton
- Lead acetate trihydrate
-

- $100.00 / 1kg
- 2024-04-25
- CAS:6080-56-4
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 20tons