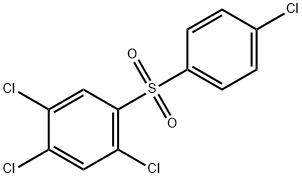
Tetradifon: Uses, Chemical properties, Toxicity, Method of production
Oct 22,2019
Chemical properties
The pure tetradifon is white crystals with no odor; the industrial tetradifon is yellow or khaki powder. M.p. :145 to 146 ° C (industrial tetradifon: 120 to 140 ° C). Vapor pressure: 3.199 × 10 -8 Pa (20 ° C). Slightly soluble in the organic solvents like ethanol, acetone, benzene, xylene and chloroform etc; insoluble in water. It is chemically stable and does not easily decompose when meeting common acid and alkali solutions, high temperature and ultraviolet light, and is also non-corrosive.
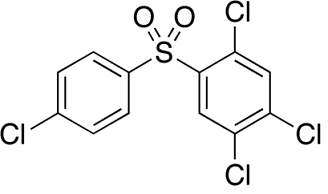
Uses
Tetradifon is used in the control of spider mites in the period of early hatching, early egg growth or fewer adult mites on cotton, citrus, apple and other plants with good effects of killing eggs and pups of mites. The longevity of residues is 40~50d. Tetradifon with the concentration of 0.05% is always used, which can inhibit the spawning of female mites and make them infertile. Tetradifon is a contact acaricide used in the control mites on fruit trees, cotton and vegetables. When tetradifon with the concentration of 0.05% is applied, it can inhibit the spawning of female mites and make them infertile.
Method of production
By chlorosulfonation: Add trichlorobenzene dropwise to chlorosulfonic acid with an excess of chlorosulfonic acid. The reaction temperature is 80-90 ° C, and the chlorosulfonation lasts 3 h. The gas discharged from the reaction is absorbed by water. Cool down the resulting trichlorobenzenesulfonyl chloride and get it precipitated and washed with water (if necessary, add a small amount of nitric acid for oxidization to convert the low-sulfur compound into trichlorobenzenesulfonyl chloride), and finally get it dehydrated and dried for condensation.
By condensation: Tetradifon is obtained by the condensation of Trichlorobenzenesulfonyl chloride and chlorobenzene. The reaction is an electrophilic second-order reaction, using AlCl3 or FeCl3 as a catalyst and adding chlorobenzene dropwise with the reaction temperature of 145-155 ° C, and the reaction time of 1.5-3 h. Then pour the condensation compound into hot water at 90 ° C, stir and separate out the chlorosulfone sulfone, and then get it washed with water and dried to obtain the original powder.
Toxicity
The acute oral LD50 (rats): >5000 mg/kg. The rats were fed with a dose of 500 mg/kg for 2 months. No harmful effects were observed. The rats were fed with 1000 mg/kg for 60 days, and no abnormalities were found in their offspring.
Reference
M. Stoeppler, Analytical chemistry of nickel. In F.W. Sunderman, ed., Nickel in the Human Environment, IARC Sci. Publ. No. 53, IARC, Lyon, France, 1989, pp. 459–468.
A. Alimonti et al., Determination of chromium and nickel in human blood by means of inductively coupled plasma mass spectrometry. Anal. Chim. Acta 306, 35–41 (1995).
M. Patriarca et al., Determination of selected nickel isotopes in biological samples by inductively coupled plasma mass spectrometry with isotope dilution. J. Anal. At. Spectrom., 11, 297–302 (1996).
- Related articles
- Related Qustion
Alloxan [2,4,5,6-tetraoxypyrimidine; 5,6-dioxyuracil] is a pyrimidine derivative of uric acid, first discovered in 1818 by Brugnaletti and then again in 1838 by Wohler.....
Oct 22,2019Biochemical EngineeringSodium cinnamate is a central intermediate in the biosynthesis of myriad natural products include lignols (precursors to lignin and lignocellulose).....
Oct 22,2019Flavors and fragrancesTetradifon
116-29-0You may like
- Tetradifon
-

- $0.00 / 25KG
- 2023-10-28
- CAS:116-29-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Tetradifon
-

- $0.00 / 1KG
- 2023-09-06
- CAS:116-29-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- Tetradifon
-

- $34.00 / 1kg
- 2023-02-08
- CAS:116-29-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000 tons