The important role of 3-(methacryloyloxy)propyltrimethoxysilane
Apr 29,2022
Background
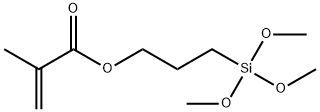
3-(Methacryloyloxy)propyltrimethoxysilane is a versatile methacryloyloxysilane, and its molecular structure has a methacryloyloxy group containing an unsaturated double bond structure functional group and three hydrolyzable methoxy functional groups[1], this dual reactivity allows it to be enhanced by bidirectional chemical reactions with inorganic materials (glass, metals, fillers) and organic polymers (thermosetting resins, plastics, elastomers) The degree of bonding, bonding and compatibility between the two, thereby improving the mechanical properties of the resin matrix composite material or improving the bonding strength and water resistance of the resin coating. At the same time, this product can also be copolymerized with acrylic acid, methacrylic acid, vinyl acetate, styrene and other monomers containing unsaturated double bonds, so as to endow the copolymer with moisture curing and good adhesion and water resistance. In different applications, it can be used as coupling agent, adhesion promoter, crosslinking agent, surface modifier for pigments and fillers, silicone modified graft comonomer, etc.
3-(Methacryloyloxy)propyltrimethoxysilane is a trimethoxysilane that hydrolyzes significantly faster than triethoxysilane SCA-R74E, which provides faster reaction and curing speed, but its hydrolysis reaction The side reactant is methanol, which is slightly less environmentally friendly. SCA-R74E has a slower hydrolysis rate and slightly better stability in aqueous systems.

Picture 1 A bottle of 3-(methacryloyloxy)propyltrimethoxysilane
Appearance and Smell
3-(Methacryloyloxy)propyltrimethoxysilane is a pale yellow to colorless clear and transparent liquid with a slightly rosin-like odor. The density (ρ20) of 3-(methacryloyloxy)propyltrimethoxysilane is 1.055g/cm3, the boiling point (760mmHg) is 255℃, the refractive index (nD25) is 1.4205. 3-(Methacryloyloxy)propyltrimethoxysilane is soluble in most aliphatic solvents such as aromatics, alcohols and ketones, and is slightly soluble in water, but hydrolyzed, and the hydrolyzate is unstable.
Reaction characteristics of 3-(methacryloyloxy)propyltrimethoxysilane
In the presence of water, 3-(methacryloyloxy)propyltrimethoxysilane is hydrolyzed to generate active silanol groups, and at the same time, methanol is released as a by-product of the hydrolysis reaction. This silanol group will undergo condensation reaction with the hydroxyl groups on the surface of various inorganic materials (substrates or pigments and fillers) to form chemical bonds; and its methacryloyloxy group can be heated or initiated by peroxides. Acrylic acid, methacrylic acid, vinyl acetate, styrene and other monomers with unsaturated double bonds are copolymerized, or the addition reaction is carried out with the newly initiated unsaturated double bonds on plastics, rubbers or resins under the action of peroxides. The resin can be fully or partially crosslinked and cured. Through the above-mentioned bidirectional reaction, this product realizes the coupling and connection between inorganic fillers (or substrates) and organic polymer materials, or realizes silanization graft modification or crosslinking of resins.
The hydrolysis of 3-(methacryloyloxy)propyltrimethoxysilane requires an organic acid (such as formic acid, acetic acid, etc.) as a catalyst. Specifically, the pH value of the water is adjusted to about 3.5~4.5, and then silane is added to stir A period of time (at least 30 minutes) until the silane is completely dissolved and the solution is clear and transparent. Their hydrolyzates are unstable, and it is recommended to use them up within 24 hours. If the solution is fogged, it means that the silane has partially self-polymerized to form a silane polymer (silicone) and fails.
3-(Methacryloyloxy)propyltrimethoxysilane can reduce the surface energy
Their aqueous solutions can significantly reduce the surface energy. For example, 0.9% aqueous solution of 3-(methacryloyloxy)propyltrimethoxysilane can reduce the surface energy by about half from 72 dynes/cm. This indicates that the hydrophobic organic part of the silane forms an alignment layer at the gas/liquid interface.
When processing mineral fillers, minerals can be blended with silanes under high shear without adding any solvent. After the silane is treated, the treated substrate or mineral surface should be briefly dried at 104-121 °C to complete the polycondensation of silanol and remove a small amount of methanol or ethanol formed during the hydrolysis of methoxysilane.
Inorganic materials suitable for 3-(methacryloyloxy)propyltrimethoxysilane include glass, glass fiber, glass wool, mineral wool, mica, quartz and other siliceous materials and aluminum hydroxide, magnesium hydroxide, kaolin, Metals such as talc, steel, zinc, and aluminum and their oxides have little effect on fillers that do not contain hydroxyl groups on the surface, such as calcium carbonate, graphite, carbon black, and barium sulfate.
3-(Methacryloxy)propyltrimethoxysilane Suitable polymers include, but are not limited to, unsaturated polyester resins, peroxide cured rubbers such as EPR, EMDP, silicone rubber, styrene-butadiene, natural, etc. Peroxide vulcanized rubber, peroxide crosslinked plastics such as PE, PVC, etc.
Use and efficacy
3-(Methacryloyloxy)propyltrimethoxysilane can improve the mechanical and electrical properties of glass fiber reinforced and inorganic filler-containing thermoset and thermoplastic resins, especially those cured by reactive radical mechanism Resins (such as unsaturated polyesters, polyurethanes, and acrylates), peroxide-cured rubbers (EPR, EPDM, silicone, etc.), and thermoplastic resins (including polyolefins and thermoplastic polyurethanes).
3-(Methacryloyloxy)propyltrimethoxysilane can significantly improve the dry and wet mechanical strength of inorganic filler-filled unsaturated polyester composites (such as cast or die-cast artificial quartz stone, etc.) and electrical performance.
3-(Methacryloyloxy)propyltrimethoxysilane can be copolymerized with vinyl acetate, acrylic acid or methacrylic acid monomers to synthesize silane-modified polymers that can be cross-linked and cured at room temperature. Such polymers are widely used Provides excellent adhesion and durability in coatings, adhesives and sealants.
Reference
1 Shin K, Kim J J, Suh K D. A facile process for generating monolithic-structured nano-silica/polystyrene multi-core/shell microspheres by a seeded sol–gel process method[J]. Journal of colloid and interface science, 2010, 350(2): 581-585.
- Related articles
- Related Qustion
3-Methacryloxypropyltrimethoxysilane
2530-85-0You may like
3-Methacryloxypropyltrimethoxysilane manufacturers
- 3-Methacryloxypropyltrimethoxysilane
-

- $0.00 / 200KG
- 2024-04-29
- CAS:2530-85-0
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 2000mt/year
- 3 Methacryloxypropyltrimethoxysilane
-

- $80.00/ kg
- 2024-03-11
- CAS:2530-85-0
- Min. Order: 500kg
- Purity: 99
- Supply Ability: 200 tons
- 3-Methacryloxypropyltrimethoxysilane
-

- $28.00 / 1L
- 2024-01-08
- CAS:2530-85-0
- Min. Order: 1L
- Purity: 99.60%
- Supply Ability: 50tons