The synergistic effect of di-tert-butyl dicarbonate
Dec 20,2019
Di-tert-butyl dicarbonate ( Boc2O) is mainly used to introduce a tert-butoxycarbonyl (Boc) protecting group to protect an amino group (especially an amino group of an amino acid), and is one of the commonly used reagents for organic synthesis. It is widely used in the industries of peptide synthesis , medicine, cosmetics and the like. Compared with other amino protecting reagents, di-tert-butyl dicarbonate has many advantages: it is cheap, has no strong irritating odor, is less toxic to the human body, and is easy to remove after reaction, and the like [1].
In synthesis reaction, Boc2O is always used with 4-(Dimethylamino)pyridine (DMAP), which works as a couple. In some cases Boc2O is also used as an apparent dehydrating agent when it reacts with carboxylic acids, certain hydroxyl groups or with primary nitroalkanes. In the conversion of nitroalkanes by Boc2O to nitrile oxides, scientists have shown that the DMAP catalyst is essential and in its absence no reaction occurs.
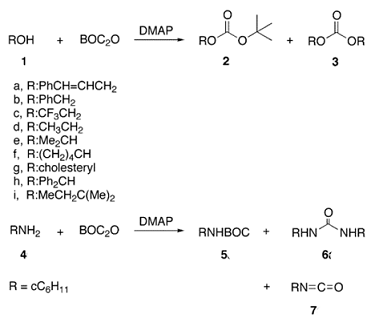
Scheme 1 Reactions of Aliphatic Alcohols and Primary Amine with Boc2O-DMAP
The efficiency of the Boc2O/DMAP couple in dehydrations of nitroalkanes prompted scientists to study reactions of other functional groups, like amines and alcohols, with Boc2O in the presence of DMAP under different conditions from the point of view of synthetic applications as well as a mechanism. Although the reactions of amines as well as of alcohols with Boc2O in the presence of DMAP are known, scientists recently found that in addition to the expected N-Boc and O-Boc derivatives other products were formed, sometimes in large amounts (Scheme 1). For instance, cinnamyl alcohol 1 reacted (in MeCN at room temperature) with Boc2O/DMAP to give the expected O-Boc derivative 2, but unexpectedly a symmetrical carbonate 3 was also isolated (ratio of 2:3 = 1:1). Furthermore, reaction of cyclohexylamine 4 with Boc2O (1.5 equiv) and DMAP (0.1 equiv) led mainly to formation of urea. The same reaction at 0 °C gave 80% of isocyanate 7. Formation of isocyanates and ureas from reaction of primary amines with Boc2O/DMAP was reported by Knölker and co-workers.
Since Boc2O and DMAP are widely used for protection of substrates that contain amine and alcohol functional groups, scientists decided to establish the major products as well as side products that can be formed in such reactions and if possible find reaction conditions that will reduce or totally prevent the formation of unwanted products.
reference
[1]https://zh.wikipedia.org/wiki/%E4%BA%8C%E7%A2%B3%E9%85%B8%E4%BA%8C%E5%8F%94%E4%B8%81%E9%85%AF
[2] Yochai Basel, Alfred Hassner, Di-tert-butyl Dicarbonate and 4-(Dimethylamino)pyridine Revisited. Their Reactions with Amines and Alcohols, J. Org. Chem. 2000, 65, 20, 6368-6380
- Related articles
- Related Qustion
- Di-tert-butyl dicarbonate: a versatile carboxylating reagent Apr 15, 2024
Di-tert-butyl dicarbonate (Boc-anhydride) is a widely used reagent in organic chemistry. It is an extremely efficient reagent to introduce the tert-butoxycarbonyl (BOC) protecting group for the amine functionality.
- Di-tert-butyl dicarbonate: Application, synthesis and toxicity Apr 14, 2023
Di-tert-butyl dicarbonate is referred to as Boc anhydride, which can be used in the protection and deprotection of amines.
Ethanesulfonic acid is an alkanesulfonic acid in which the alkyl group directly linked to the sulfo functionality is ethyl [1], and it is a conjugate acid of an ethanesulfonate. employed in the electrolytic reduction of perrhenate solutions....
Dec 20,2019Chemical ReagentsTests on rodents suggest L-alanyl-L-glutamine is more successful at increasing muscular glutamine levels after supplementation than glutamine itself. This is because glutamine is absorbed better via the liver and intestines after supplement....
Dec 20,2019Biochemical EngineeringDi-tert-butyl dicarbonate
24424-99-5You may like
- Is L-carnitine helpful in losing weight?
May 15, 2024
- Is DSIP Delta-Sleep-Inducing Peptide Safety?
May 15, 2024
- How to prevent high Cholesterol levels in daily life?
May 11, 2024
Di-tert-butyl dicarbonate manufacturers
- Di-Tert-Butyl Dicarbonate
-

- $0.00 / 1kg
- 2024-05-15
- CAS:24424-99-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons
- Di-Tert-Butyl Dicarbonate
-

- $0.00 / 1kg
- 2024-05-15
- CAS:24424-99-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons
- Di-tert-butyl dicarbonate
-

- $0.00 / 25kg
- 2024-05-15
- CAS:24424-99-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons




