The synthesis method of Diethylene glycol vinyl ethers
Mar 19,2024
Reaction
Acetylene reacts with diethylene glycol in the presence of a catalyst such as potassium hydroxide or potassium diethylene glycol to yield Di(ethylene glycol) vinyl ether (DEGMVE) and Diethylene glycol divinyl ether (DEGDVE):
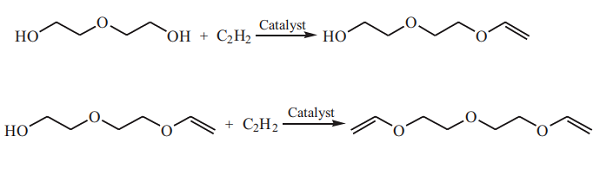
The Acetylene route has advantages, including a simple process, cheap and easily obtained raw materials, low production cost, and fewer by-products. However, the reaction must be carried out at elevated temperature and pressure, and acetylene gas is flammable and explosive.
Method
Zhang et al. prepared diethylene glycol vinyl ethers in a packed-bed reactor at a temperature of 175 °C and under a pressure of more than 0.6 MPa. The catalyst used was potassium diethylene glycol prepared from metal potassium, and the potassium concentration was 2% based on the mass of diethylene glycol. The gas–liquid reaction of acetylene and diethylene glycol was continuously carried out at an acetylene space velocity of 0.3–0.4 h−1. The conversion of the diethylene glycol reached 75%, and the overall yield of diethylene glycol vinyl ethers was 72%. Zhao et al. used a bubbling-bed reactor to carry out the gas–liquid reaction of acetylene and diethylene glycol for batch preparation of diethylene glycol vinyl ethers under atmospheric pressure. Potassium diethylene glycol was used as the catalyst; the concentration was 10%. After reaction for 8 h at a temperature of 175 °C and acetylene flow rate of 35 mL · min−1, the conversion of diethylene glycol was 63.7%, and the total yield of diethylene glycol vinyl ethers was 61.2%.
Li's research group at Xiamen University developed a process for continuous production of diethylene glycol vinyl ethers from acetylene and diethylene glycol by full liquid-phase cyclic reaction in a tubular reactor. Pure acetylene gas was dissolved in the diethylene glycol solution containing 4% of potassium diethylene glycol at low temperature and low pressure in advance. Then the resulting glycol solution saturated with acetylene was pressurized by a plunger metering pump and introduced into a tubular reactor for full liquid-phase reaction at a temperature of 175 °C and a pressure of 6 MPa. The residence time of the reaction solution in the reactor was 175 s. The outlet solution from the reactor was cooled and decompressed by a pressure relief valve, a small portion of the solution was discharged as the product, and the rest of the solution was circulated back to an absorber to absorb acetylene together with the feed liquid (diethylene glycol and catalyst). Then the solution was pressurized and introduced into the tubular reactor for continuous reaction. At steady state, the conversion of diethylene glycol reached 76.03%, and the total yield of diethylene glycol vinyl ethers was up to 74.13%, in which the yield of DEGMVE was 59.03%, and the yield of DEGDVE was 15.10%. Because of gas-phase acetylene in the tubular reactor, the danger of flammable and explosive acetylene in the gas phase under high temperature and high pressure has been overcome.
Separation
The reaction solution discharged from the reactor was a mixture of diethylene glycol, potassium diethylene glycol, DEGMVE, and DEGDVE. Therefore, a separation process was required to obtain pure diethylene glycol mono vinyl ether and pure diethylene glycol divinyl ether. Since the boiling points between DEGMVE and DEGDVE are not much different, fractional distillation requires a high column with larger theoretical plates. However, DEGMVE and DEGDVE form an azeotrope, and pure DEGDVE cannot be obtained from the column top, regardless of the reflux ratio and the number of theoretical plates. The reaction mixture was subjected to distillation under vacuum. The azeotrope of DEGMVE and DEGDVE was distilled off at 133 °C and 6 kPa. The mass fraction of DEGDVE in the azeotrope was about 25%. Subsequently, the temperature was increased to 141 °C to distill off the residual DEGMVE. The separation of the azeotrope of DEGMVE and DEGDVE was performed by adding potassium hydroxide to the azeotrope for binding DEGMVE. Then the mixture was subject to distillation for pure DEGDVE. Another separation method for the reaction mixture is extractive distillation.
- Related articles
- Related Qustion
It’s usually safe to drink. Some people drink baking soda for indigestion and other purposes, but drinking baking soda can be dangerous and is not suitable for long-term use, use during pregnancy, or use in children.....
Mar 19,2024Inorganic chemistryDry ice is the solid form of carbon dioxide (chemical formula CO2), comprising two oxygen atoms bonded to a single carbon atom.....
Mar 19,2024Inorganic chemistryDiethylene glycol divinyl ether
764-99-8You may like
- The solubility of Succinic anhydride
May 11, 2024
- How to synthesize Benzyl vinylcarbamate?
Mar 26, 2024
- Polypropylene and Polyvinyl chloride: Which one is better?
Mar 21, 2024
Diethylene glycol divinyl ether manufacturers
- Diethylene glycol divinyl ether
-

- $20.00 / 1kg
- 2023-10-14
- CAS:764-99-8
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Diethylene glycol divinyl ether
-

- $20.00 / 1kg
- 2022-09-03
- CAS:764-99-8
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 5000 tons
- Diethylene glycol divinyl ether
-

- $1.00 / 1kg
- 2019-07-06
- CAS:764-99-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: Customized






