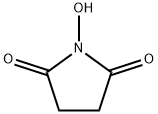
The Use of Esters of N-Hydroxysuccinimide in Peptide Synthesis
Nov 5,2019
In recent years, p-nitrophenyl esters of acylamino acids have been of great value in the synthesis of peptide derivatives. Other activated esters such as cyanomethyl esters have been promising, and most recently esters of N-hydroxyphthalimide4ab have been shown to be valuable intermediates. The latter are particularly interesting because of their high reactivity, and in addition they are readily crystallized. It seemed worthwhile to us to explore the field of hydroxylamine compounds for derivatives with still better properties. We are particularly interested in derivatives which give readily removable by-products; in the field of simple hydroxylamine derivatives, water solubility appeared to be an attractive possibility. In contrast to N-hydroxyphthalimide, N-hydroxysuccinimide5 is water soluble, and we have found that acylamino acid esters of this compound meet the important requirements of crystallinity and high reactivity. Consequently we have undertaken an extensive investigation of these derivatives.
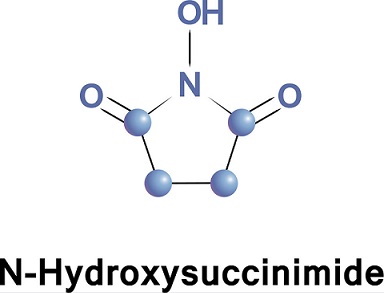
N-Hydroxysuccinimide has previously been best prepared via the O-benzyl derivative6 or by fusion of succinic anhydride with hydroxylamine. ‘We have employed a simplification of the latter procedure using hydroxylamine hydrochloride to give a 44% yield of pure N-hydroxysuccinimide, with a melting point of 99-100°.
The dicyclohexylcarbodiimide method of ester synthesis, which has been used for the synthesis of esters of N-hydroxyphthalimide4 and p-nitrophenyl esters,has been used for the synthesis of the N-hydroxysuccinimide esters of a number of N-protected amino acids. These are colorless crystalline derivatives with good stability . Exposure to the atmosphere for weeks or months resulted in slight lowering of the melting points in some cases, but storage of the pure compounds in a desiccator gave no change in melting points in 2 months.
Acylpeptide esters were conveniently prepared by the reaction of N-hydroxysuccinimide derivatives with equivalent amounts of amino acid or peptide esters in organic solvents at room temperature. Usually a 40-min. reaction time was used (a longer period may improve yields in some cases) and the peptide product was precipitated by the addition of water. Examples 6 and 7 (Experimental), illustrate the procedure used.
It was of interest to try reactions under aqueous conditions, since older methods of peptide synthesis usually give poorer yields in the presence of water. Illustrative results are given in examples. Several experiments in the synthesis of Z-gly-L-tryOH (example 11) showed that the use of 2 equivalents of sodium bicarbonate is desirable. Examples 8B and 9B indicate that the analogous use of esters of N-hydroxyphthalimide gave poor results largely because of the water insolubility of N-hydroxyphthalimide.
The results with the aqueous reactions are promising for the development of conditions for the lengthening of peptide chains by the reaction of esters of N-hydroxysuccinimide with salts of peptides or proteins. Further work in this direction is projected.
Experimental
Melting points were determined on a calibrated Fisher-Johns block.
1. N-Hydroxysuccinimide.— -Succinic anhydride (100 g., 1.0 mole) and hydroxylamine hydrochloride were combined in a 1-1. flask. The flask was placed on a rotary evaporator and the contents heated by a silicone oil bath. The volatile products which formed were removed under vacuum provided by a water aspirator and, after passage through a cold water condenser, were caught in a Dry Ice trap. The contents of the flask were rapidly heated to about 125° where fusion occurred with evolution of gases. Over the next hour the temperature was increased slowly up to 160°, at which point the formation of water had virtually ceased. The heating was discontinued. When the temperature had dropped to 125°, the amber liquid was poured into a beaker containing 400 ml. of ether which was vigorously stirred. The ether layer was decanted after the product solidified and the residue heated to boiling with 400 ml. of dry 1-butanol. The mixture was filtered and the filtrate rapidly chilled to 0°. After a 1-hr. period, the crystalline material was collected by filtration and the residue was washed carefully with 1-butanol, then ether. The crude product, melting at 93 to 95°, amounted to 75 g.
2. N-Hydroxysuccinimide Ester of Benzyloxycarbonylglycine. —N,N-Dicyclohexylearbodiimide (26 g., 0.126 mole) was added to a solution of benzyloxycarbonylglycine (26.3 g., 0.126 mole) and 14.5 g. (0.126 mole) of hydroxysuccinimide in 250 mi. of dioxane with cooling. The reaction mixture was allowed to stand in the refrigerator overnight. The formed dicyclohexylurea was filtered and washed with dioxane. The filtrate was concentrated in vacuo to yield a yellow oil which soon crystallized. The compound was triturated with ether and filtered to obtain 37.5 g. (97%), m.p. 108-11°. Recrystallization from methylene chloride and petroleum ether yielded 33 g. (86%), m.p. 113-114°.
3. N-Hydroxysuccinimide Ester of /-Butyloxycarbonyl-i.- alanine.—Butyloxycarbonyl-L-alanine15 (1.32 g., 0.0070 mole) and N-hydroxysuccinimide (0.805 g., 0.0070 mole) were mutually dissolved in 10 ml. of anhydrous dimethoxyethane at 0°. Then dicyclohexylcarbodiimide (1.59 g., 0.0070 mole + 10%) was dissolved with stirring and the solution kept at 0-5° for a period of 20 hr.
The urea which formed was separated by filtration and the filtrate evaporated to dryness in an open dish leaving a crystalline residue of 2.33 g. of crude product. Two successive recrystallizations from isopropyl alcohol gave the pure product, 1.42 g. (71%), melting at 143-144°. The other compounds in Table II were similarly prepared.
4. N-Hydroxysuccinimide Ester of Phthaloylglycine.— Phthaloylglycine16 (4.10 g., 0.020 mole) and hydroxysuccinimide (2.30 g., 0.020 mole) were dissolved together in 25 ml. of anhydrous dimethoxyethane. To the ice-cold solution was added dicyclohexylcarbodiimide (4.53 g., 0.020 mole + 10%) with stirring. After a period of 20 hr. at 0° the urea was removed by filtration and the filtrate concentrated to dryness by evaporation of the solvent in an open vessel. The residue was recrvstallized from isopropyl alcohol to yield the analytically pure product, m.p. 182-183°, yield 4.21 g. (70%). Anal. Caled, for C„H10N2O6; C, 55.63; H, 3.34; N, 9.27. Found; C, 55.86; H, 3.36; N.
5. N-Hydroxysuccinimide Ester of Tritylglycine.—Tritylglycine17 (6.34 g., 0.020 mole) and hydroxysuccinimide (2.30 g., 0.020 mole) were mutually dissolved in 125 ml. of anhydrous dimethoxyethane (solvent). The solution was cooled to 5° and dicyclohexylcarbodiimide (4.52 g., 0.020 mole +10%) added. After a period of 20 hr. at 5°, the urea was separated by filtration and the filtrate evaporated to dryness leaving a residue of 8.85 g. Recrystallization from isopropyl alcohol yielded 6.90 g. (83%) of the pure product, m.p. 145.5-146.5°. Anal. Caled, for C26H22N204: C, 72.45; H, 5.35; N, 6.76. Found: C, 72.54; H, 5.39; N, 6.95.
6. Ethyl Benzyloxycarbonyl-L-phenylalanyl-L-tyrosinate.—A solution of 1.98 g. (0.005 M) of the N-hydroxysuccinimide ester of carbobenzoxy-L-pheuylalanine in 10 ml. of dimethoxyethane was added to a solution of 1.045 g. (0.005 M) of ethyl tyrosinate18 (l) in 10 ml. of the same solvent. The reaction was allowed to stand for 40 min. at room temperature and was then diluted with 60 ml. of water. The crystals which formed immediately were collected and washed with 10% sodium bicarbonate solution, water, N hydrochloric acid, and water. The crude yield after drying was 2.62 g. (100%), m.p. 151-157°. Two recrystallizations from ethanol-water yielded 2.08 g. (85%), m.p. 156-158°, [a]26d —9.1 ± 0.5° (c 10.0, ethanol). These results compare favorably with the literature values of m.p. 159-160°, [«]24d — 9.1 ± 0.1° (e 10, ethanol), and 46% yield by the mixed anhydride procedure.
7. Ethyl t-Butyloxycarbonylglycyl-L-phenylalanylglycinate.— Ethyl L-phenylalanylglvcinate hydrobromide (2.48 g., 75 mmoles), the hydroxysuccinimide ester of i-butyloxycarbonylglycine (2.04 g., 75 mmoles), and triethylamine (1.05 ml., 75 mmoles) were mutually combined in 25 ml. of anhydrous dimethoxyethane. The mixture was stirred for a period of 20 min. after which it was added to 100 ml. of cold water. The product crystallized and was collected by filtration. The crude product (2.58 g.) melted at 98-99°. Recrystallization from alcoholwater yielded the pure tripeptide, m.p. 100.5-101.5°, with a net yield of 79%; [a]25d —9.75 ± 2.46° (c 2.05, methanol). Anderson and McGregor16 reported a yield of 57% by the pyrophosphite method and a melting point of 98-99°
- Related articles
- Related Qustion
- N-Hydroxysuccinimide and N-hydroxysuccinimide ester Apr 26, 2024
N-Hydroxysuccinimide, commonly known as NHS, is an intermediate used in peptide chemistry to prepare various activated esters of acylamino acids.
- N-Hydroxysuccinimide: Synthesis, application and pharmacokinetics Apr 17, 2023
N-Hydroxysuccinimide (NHS) is an organic compound to used to prepare active esters in organic synthesis.
The imidazole group is an ubiquitous chemical motif present in several key types of biomolecules. It is a structural moiety of purines, and plays a central role in biological catalysis as part of the side-chain of histidine, the amino acid....
Nov 5,2019Chemical pesticides ?p-Toluenesulfonic acid (PTSA or pTsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents.....
Nov 5,2019Organic ChemistryN-Hydroxysuccinimide
6066-82-6You may like
- Nisin: Application, Mechanism of action and Side effects
Apr 28, 2024
- What is the primary function of NAD and FAD?
Apr 28, 2024
- The benefits of Copper tripeptide
Apr 22, 2024
N-Hydroxysuccinimide manufacturers
- N-Hydroxysuccinimide
-

- $30.00 / 1kg
- 2024-04-28
- CAS:6066-82-6
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
- N-Hydroxysuccinimide
-

- $0.00 / 25Kg/Drum
- 2024-04-28
- CAS:6066-82-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500kg
- N-Hydroxysuccinimide
-
- $0.00 / 10mg
- 2024-04-11
- CAS:6066-82-6
- Min. Order: 10mg
- Purity: 98.69%
- Supply Ability: 10g/month