AZD3759- preclinical and clinical studies
Dec 13,2019
AZD3759 is a selective EGFR inhibitor that can fully penetrate the blood-brain barrier (BBB), with equal free concentrations in the blood, cerebrospinal fluid, and brain tissue. Treatment with AZD3759 causes tumor regression in subcutaneous xenograft, leptomeningeal metastasis (LM), and brain metastasis (BM) lung cancer models and prevents the development of BM in nude mice.
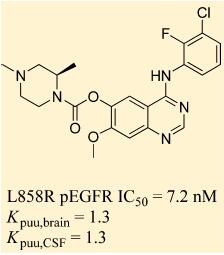
Preclinical studies of AZD3759 were performed with erlotinib as a comparator. In murine models of brain metastasis and LM, AZD3759 (15/mg/kg) exhibited excellent CNS penetration, and achieved concentrations above the pEGFR inhibitory concentration (IC50) for longer than 7 h, compared with 6 h or less for erlotinib. AZD3759 was shown to cause tumor regression, and survival rates were significantly higher than those with erlotinib [1].
Currently, AZD3759 is undergoing clinical evaluation in the phase I BLOOM study, which is assessing both AZD3759 and the third-generation EGFR TKI osimertinib treatment in heavily pretreated patients with EGFR mutation-positive NSCLC who had progressed on prior EGFR TKI therapy and had a confirmed diagnosis of LMD [2]. Patients had mostly good ECOG PS (57% ECOG 0/1) and 48% had neurological symptoms. AZD3759 has been found to be well tolerated in patients with LMD previously treated with at least one line of EGFRTKI therapy and chemotherapy. One patient (6%) discontinued treatment due to an AE (skin disorder), and some antitumor activity has been observed at an AZD3759 dose of 200–300 mg twice daily [3]. In this study, 5 out of 18 patients (28%) had a confirmed PR or CR in the brain, and 9 (50%) had a SD. Only 6 of 18 patients (33%) with extracranial lesions had tumor shrinkage, with no confirmed PR. Two case studies reported similar AZD3759 trough plasma and CSF concentrations 1 week after the start of treatment, indicating full penetration of the BBB [1]. BLOOM is also assessing AZD3759 in patients with treatment-naïve CNS manifestation. Twenty EGFR mutation-positive NSCLC patients, with either brain metastases (n = 16) or leptomeningeal metastasis (n = 4; three pretreated with WBRT) were treated with AZD3759 (200 or 300mg twice daily). Fifty-five percent experienced grade ≥ 3 AEs (30% skin-related, 20% gastrointestinal), and the treatment discontinuation rate was 10%. Efficacy data were encouraging, with 15 (83%) patients with measurable brain metastases achieving an objective response (1 complete response); 3 (75%) patients with LMD also achieved an objective response, as did 13 (72%) patients with extracranial manifestations (2 complete responses) [4].
In summarize, an early clinical study in patients with BM and LM treated with AZD3759 confirms its BBB-penetrant properties and antitumor activities. The clinical study data demonstrate the potential of AZD3759 for the treatment of BM and LM and support its further clinical evaluation in larger trials.
References
1.Yang Z, Guo Q, Wang Y, Chen K, Zhang L, Cheng Z. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases[J]. Sci Transl Med. 2016, 8(368):368ra172.
2.Yang JC-H, Cho BC, Kim D-W, Kim S-W, Lee J-S, SuW-C. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC):Updated results from the BLOOM study[J]. J Clin Oncol. 2017, 35(suppl 18):abst 2020.
3.Cho BC, Ahn M-J, Lee J-S, Kim D-W, Kim S-W, John T. Phase I study (BLOOM) of AXD3759, a BBB penetrable EGFR inhibitor, in EGFRm NSCLC patients with leptomeningeal metastasis (LM) who progressed after other anti-cancer therapy[J]. J Clin Oncol. 2017, 35(suppl 18):abst 2069.
4.Ahn M-J, Kim D-W, Cho BC, Kim S-W, Lee J-S, Ahn JS. Phase I study (BLOOM) of AXD3759, a BBB penetrable EGFR inhibitor, in TKI naïve EGFRm NSCLC patients in CNS metastases[J]. J Clin Oncol. 2017, 35(suppl 18):abst 2006.
- Related articles
- Related Qustion
- The Applications and Mechanism of Action of AZD 3759 Mar 6, 2024
AZD 3759 is a novel inhibitor targeting EGFR tyrosine kinase, which has been used in clinical treatment and other aspects.
- AZD 3759: Pharmacodynamics, Mechanism of action, Toxicity, Preparation Apr 12, 2023
AZD3759 is a small molecule inhibitor of the anaplastic lymphoma kinase (ALK) receptor tyrosine kinase. It is being developed as a potential treatment for non-small cell lung cancer.
Neratinib is an oral pan HER inhibitor that irreversibly inhibits the tyrosine kinase activity of epidermal growth factor receptors, EGFR (or HER1), HER2, and HER4.....
Dec 13,2019InhibitorsLarotrectinib (VITRAKVIR) is an orally administered, small molecule, highly-selective, tropomyosin receptor kinase (TRK) inhibitor that was developed by Loxo Oncology in collaboration with Bayer AG.....
Dec 13,2019InhibitorsAZD 3759
1626387-80-1You may like
- AZD3759
-

- $0.00 / 1kg
- 2023-10-09
- CAS:1626387-80-1
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20tons
- AZD 3759
-

- $100.00 / 25kg
- 2023-01-31
- CAS:1626387-80-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000KG/month
- AZD 3759
-

- $0.00 / 1g
- 2022-05-18
- CAS:1626387-80-1
- Min. Order: 10g
- Purity: 99.0%
- Supply Ability: 10tons