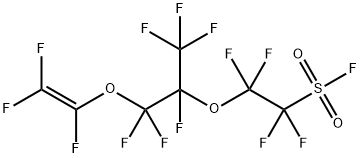
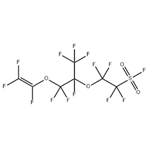
Application of Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride in electrolyte membrane material
Feb 26,2020
The rapid global warming caused by the use of fossil fuel sources incites investigation ofalternative energy production processes and sources. Among energy conversion devices,polymer electrolyte membrane fuel cells (PEMFC) are under consideration for automotive,portable and stationary applications. So far, most advanced PEMFC use perfluorosulfonic acids (PFSAs) as the electrolyte membrane[1]. PFSA membranes exhibit high protonconductivity, as well as high chemical, thermal, oxidative, and mechanical stabilities.However, the push towards ever higher fuel cell performance and consequently the use of lowequivalent weight ionomers means that additional measures are required to further improvemembrane mechanical properties[2]. Most commonly, a reinforcement material is used,however alternatively, incorporation of inorganic networks or particles, or polymer chaincrosslinking, are used.
Covalent crosslinking, required to enhance chemical, physical, mechanical and thermalproperties, is a technique that enables the stabilization of ionomer membranes, hence decreasingtheir volumetric swelling and increasing their mechanical robustness. This strategy is widely used to improve the mechanical properties of non-fluorinated polymers.Nevertheless, several studies have been reported on the crosslinking of PFSA ionomers. Crosslinking of PFSA generally requires either the conversion of the sulfonic acid side chainsor the synthesis of a polymer containing crosslinkable functional groups[3]. This strategy was firstdescribed in the late 1970s. Nafion® was first chemically modified by convertingits sulfonic acid side chains into sulfonyl chloride groups and then the crosslinking wasperformed by treatment with ethylene diamine to allow the formation of sulfonamides. Thermalpost-treatment was required to promote the crosslinking. Grot disclosed a simplecondensation between a sulfonyl fluoride and a sulfonamine which led to a strong sulfonamidefunction. This method was further investigated by Lousenberg (DuPont) and Uematsu etal[4].
Irradiation using x-rays or y-rays, charged particles, electron beam or ions of heavy metals can also effect crosslinking. The most commonly used source of y-rays is Co60 that emits radiation ranging from 1.17 to 1.33 MeV. Sol-gel reactions can also be used to crosslink polymer membranes. This strategy was used with telechelic fluorinated copolymersbearing bis(trialkoxysilane) or vinyltriethoxysilane units[5]. Other crosslinkingstrategies for fluorinated polymers are based on reinforced composites such as thoseprepared from Nafion® and crosslinkable fluorinated polyimides or polybenzimidazole, photocrosslinking (from azides by formation of nitrenes) semi interpenetrated polymer network (for example combining perfluorosulfonicacid ionomer with a perfluoropolyether network, in the presence ofdivinylbenzene-PVDF followed by sulfonation) from a fluoropolymer that bears 1H 1,2,4-triazole groups and s-PEEK and the use of cure site monomers. Usually, acure site monomer (CSM) is incorporated into a copolymer to allow crosslinking in a postpolymerisation step. Among CSMs, trifluorovinyl monomers bearing bromine atoms havebeen used to crosslink membranes based on VDF. Perfluoro(8-cyano-5-methyl-3,6-dioxaoct-1-ene) (CNVE), 2,2,3,3,4,4,5,5,6,6-decafluoro-6-[(1,2,2-trifluoroethenyl)oxy] hexanenitrile MV5CN were successfully used in terpolymers to allow the crosslinking (by formation of stable triazine rings) of fluorinated elastomers[6]. These nitrile bearingperfluorinated elastomers exhibit outstanding oxidative and thermal stabilities andresearch efforts have been focused to broaden the areas in which these polymers can be used. Crosslinked terpolymers of vinylidene fluoride, perfluoro-3,6-dioxa-4-methyl-7-octene sulfonyl fluoride, and cure site monomers, and the thermal crosslinking of the hydrolysed resulting materials into proton conducting membranes, for potential use in PEMFC[7].
References
[1] A. S. Odinokov, O. S. Bazanova, L. F. Sokolov. Kinetics of Copolymerization of Tetrafluoroethylene with Perfluoro(3,6-dioxa-4-methyl-7-octen)sulfonyl Fluoride[J]. Russian Journal of Applied Chemistry, 2009, 82(1):112-115.
[2] Colpaert, Maxime, Zaton, Marta, Ladmiral, Vincent. Crosslinked terpolymers Based on Vinylidene fluoride, perfluoro- 3,6-dioxa-4-methyl-7-octene sulfonyl fluoride, and cure site monomers for membranes in PEMFC applications.[J]. Polymer Chemistry.
[3] Ozlem Sel, Aurélien Soulès, Bruno Améduri. Original Fuel-Cell Membranes from Crosslinked Terpolymers via a “Sol–gel” Strategy[J]. 20(7):1090-1098.
[4] L. Sauguet, B. Ameduri, B. Boutevin. Fluorinated copolymers and terpolymers based on vinylidene fluoride and bearing sulfonic acid side-group [J]. Journal of Polymer Science Part A Polymer Chemistry, 2007, 45(10):1814-1834.
[5] AI Aleksandrov, AI Prokofev, V. N. Lebedev. Preparation of ultrafine metal particles in processes of pulse mechanical action[J]. Russian Chemical Bulletin, 1995, 44(12):2251-2254.
- Related articles
- Related Qustion
Donepezil hydrochloride is a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as (±)-2, 3-dihydro-5, 6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one hydrochloride.....
Feb 26,2020InhibitorsMethylglyoxal is a highly reactive alpha-oxoaldehyde formed endogenously in numerous enzymatic and nonenzymatic reactions.....
Feb 26,2020Organic reagentsYou may like
Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride manufacturers
- Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
-
- $44.00 / 5g
- 2024-02-23
- CAS:16090-14-5
- Min. Order: 5g
- Purity: 0.99
- Supply Ability: 25kg
- Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
-
- $5.00 / 1KG
- 2024-01-14
- CAS:16090-14-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
-
- $0.00 / 1KG
- 2022-10-09
- CAS:16090-14-5
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1Ton