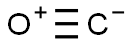Carbon monoxide-Health Hazard and Toxicity
Sep 11,2019
Carbon monoxide is a colorless, odorless, tasteless, flammable, toxic gas.Carbon monoxide is produced when carbon and carbon compounds undergo incomplete combustion. The inefficient combustion of carbon fuels for heating results in the production of carbon monoxide, which may result in high CO concentrations in indoor environments.
It was first identified by the Spanish alchemist Arnold of Villanova (1235–1313), who noted the production of a poisonous gas when wood was burned. The formal discovery of carbon monoxide is credited to the French chemist Joseph Marie Fran?ois de Lassone (1717–1788) and the British chemist Joseph Priestley (1733–1804). The former prepared carbon monoxide by heating carbon in the presence of zinc, and for a time the compound was incorrectly identified as hydrogen. William Cumberland Cruikshank (1745–1800) correctly determined that carbon monoxide was an oxide of carbon in 1800.

Major Hazards
Moderately toxic gas with no warning properties; decreases the ability of the blood to carry oxygen to the tissues.
Toxicity
The acute toxicity of carbon monoxide by inhalation is moderate. Carbon monoxide is a chemical asphyxiant that exerts its effects by combining preferentially with hemoglobin, the oxygen-transport pigment of the blood, thereby excluding oxygen. Symptoms of exposure to CO at 500 to 1000 ppm include headache, palpitations, dizziness, weakness, confusion, and nausea. Loss of consciousness and death may result from exposure to concentrations of 4000 ppm and higher; high concentrations may be rapidly fatal without producing significant warning symptoms. Exposure to this gas may aggravate heart and artery disease and may cause chest pain in individuals with preexisting heart disease. Pregnant women are more susceptible to the effects of carbon monoxide exposure. Since carbon monoxide is odorless, colorless, and tasteless, it has no warning properties, and unanticipated overexposure to this highly dangerous gas can readily occur.
Carbon monoxide has not been found to be carcinogenic in humans. This substance has shown developmental toxicity in animal tests. Chronic exposures to carbon monoxide at levels around 50 ppm are thought by some investigators to have a negative impact on the results of behavioral tests such as time discrimination, visual vigilance, choice response tests, visual evoked responses, and visual discrimination thresholds.
Flammability and Explosibility
Carbon monoxide is a flammable gas. It forms explosive mixtures with air in the range of 12.5 to 74% by volume.
Reactivity and Incompatibility
Carbon monoxide is a reducing agent; it reacts violently with strong oxidizers. It undergoes violent reactions with many interhalogen compounds such as ClF3, BrF3, and BrF5. CO reacts with many metals to form metal carbonyls, some of which may explode on heating, and reduces many metal oxides exothermically. Carbon monoxide reacts with sodium and with potassium to form explosive products that are sensitive to shock, heat, and contact with water.
Storage and Handling
Because of its toxic, flammable, and gaseous nature, carbon monoxide should be handled using the ''basic prudent practices" of Chapter 5.C, supplemented by the additional precautions for work with flammable compounds (Chapter 5.F) and for work at high pressure (Chapter 5.H). In particular, cylinders of carbon monoxide should be stored and used in a continuously ventilated gas cabinet or fume hood. Local fire codes should be reviewed for limitations on quantity and storage requirements.
Accidents
In the event of a release of carbon monoxide, evacuate the area immediately. Rescue of an affected individual requires appropriate respiratory protection. Remove exposed individual to an uncontaminated area and seek immediate emergency help. Keep victim warm, quiet, and at rest and provide assisted respiration if breathing has stopped.
To respond to a release, use appropriate protective equipment and clothing. Positive pressure air-supplied respiratory protection is required. Close cylinder valve and ventilate area. Remove cylinder to a fume hood or remote area if it cannot be shut off.
Disposal
Excess carbon monoxide should be returned to the manufacturer, according to your institution's waste disposal guidelines.
Causes
Carbon monoxide poisoning is caused by inhaling combustion fumes. When too much carbon monoxide is in the air you're breathing, your body replaces the oxygen in your red blood cells with carbon monoxide. This prevents oxygen from reaching your tissues and organs.
Various fuel-burning appliances and engines produce carbon monoxide. The amount of carbon monoxide produced by these sources usually isn't cause for concern. But if they're used in a closed or partially closed space — cooking with a charcoal grill indoors, for example — the carbon monoxide can build to dangerous levels.
Risk factors
Exposure to carbon monoxide may be particularly dangerous for:
Unborn babies. Fetal blood cells take up carbon monoxide more readily than adult blood cells do. This makes unborn babies more susceptible to harm from carbon monoxide poisoning.
Children. Young children take breaths more frequently than adults do, which may make them more susceptible to carbon monoxide poisoning.
Older adults. Older people who experience carbon monoxide poisoning may be more likely to develop brain damage.
People who have chronic heart disease. People with a history of anemia and breathing problems also are more likely to get sick from exposure to carbon monoxide.
Those in whom carbon monoxide poisoning leads to unconsciousness. Loss of consciousness indicates more severe exposure.
Complications
Depending on the degree and length of exposure, carbon monoxide poisoning can cause:
Permanent brain damage
Damage to your heart, possibly leading to life-threatening cardiac complications
Fetal death or miscarriage
Death
- Related articles
- Related Qustion
- Is Carbon Monoxide Polar or Nonpolar? Dec 21, 2023
Carbon Monoxide is a diatomic molecule, the partial negative charge exists on the carbon and partial positive charge exists on the oxygen atom.These electronic exchange between carbon and oxygen atoms make the CO a polar molecule.
- How to draw the Lewis structure of CO Nov 17, 2023
The Lewis structure of CO is made up of a carbon atom (C) and an oxygen atom (O). The carbon and oxygen atoms are connected by a triple bond with a lone electron pair on each atom.
- Biological functions of Carbon monoxide Jun 24, 2022
Carbon monoxide is the second gasotransmitter that was found to have a physiological role in neurotransmission, cardiovascular regulation, and oxygen sensing.
Brigatinib (marketed as Alunbrig) is a small-molecule targeted cancer therapy being developed by ARIAD Pharmaceuticals, Inc. Brigatinib acts as both a anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor. B....
Sep 11,2019APICarbon tetrachloride, also known as tetrachloromethane, has its molecule formula being CCl4. It appears as colorless liquid with the melting point of-23 ° C, boiling point of 76.8 ° C and the relative density of 1.5867. It can dissolve grea....
Sep 11,2019Organic ChemistryCARBON MONOXIDE
630-08-0You may like
- Crystal Structure of Aluminum Selenide
Apr 23, 2024
- Crystal Structure and Property of Tin Selenide
Apr 23, 2024
- Crystal Structure of Uranium Carbide
Apr 22, 2024
- CARBON MONOXIDE
-

- $15.00 / 1KG
- 2021-08-12
- CAS:630-08-0
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton