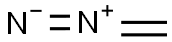Diazomethane - Health Hazard and Toxicity
Sep 12,2019
Diazomethane has a molecular weight of 42.041 g/mol, and a monoisotopic mass of 42.022 g/mol, which is also its exact mass. Diazomethane has a musty odor, and it is shipped in the form of compressed gas.Diazomethane can be manufactured from an alkali (potassium carbonate or potassium hydroxide) reaction With N-Nitrosomethyl-P-Toluenesulfonamide. It can also be prepared from ether solutions of diazomethane, where N-nitroso-beta-methylaminoisobutyl methyl ketone in isopropanol and ether is reacted with sodium isopropoxide.
Gaseous diazomethane is prepared from hydrazine and chloroform through a reaction with potassium hydroxide or from nitrosomethylurea and potassium hydroxide.

Toxicity Data
LCLO inhal (cat)
175 ppm (10 min)
PEL (OSHA)
0.2 ppm (0.4 mg/m3)
TLV-TWA (ACGIH)
0.2 ppm (0.4 mg/m3)
Major Hazards
Powerful allergen with high acute toxicity; extremely unstable; may explode on contact with alkali metals, calcium sulfate (Drierite), or rough edges such as those found on ground glass.
Toxicity
Diazomethane vapor causes severe irritation of the skin, eyes, mucous membranes, and lungs. It is considered to be a substance with poor warning properties, and the effects of exposure may be delayed in onset. Symptoms of exposure may include headache, chest pain, cough, fever, severe asthmatic attacks, and pulmonary edema, which can be fatal. Exposure of the skin and mucous membranes to diazomethane may cause serious burns.
Diazomethane is a powerful allergen. Prolonged or repeated exposure to diazomethane can lead to sensitization of the skin and lungs, in which case asthmalike symptoms or fever may occur as the result of exposure to concentrations of diazomethane that previously caused no symptoms. Chronic exposure to diazomethane has been reported to cause cancer in experimental animals, but this substance has not been identified as a human carcinogen.
Note that diazomethane is often prepared in situ from precursors that may themselves be highly toxic and/or carcinogenic.
Flammability and Explosibility
Pure diazomethane gas and liquid are readily flammable and can explode easily. A variety of conditions have been reported to cause explosions of diazomethane, including contact with rough surfaces such as ground-glass joints, etched or scratched flasks, and glass tubing that has not been carefully fire-polished. Direct sunlight and strong artificial light may also cause explosions of this substance. Violent reactions may occur on exposure of diazomethane to alkali metals.
Reactivity and Incompatibility
Explosions may occur on exposure of diazomethane to alkali metals and calcium sulfate (Drierite).
Storage and Handling
Because of its high toxicity and explosibility, diazomethane should be handled using the "basic prudent practices" of Chapter 5.C, supplemented by the additional precautions for work with compounds of high chronic toxicity (Chapter 5.D) and for work with reactive and explosive substances (Chapter 5.G). In particular, diazomethane should preferably be handled in solution using glassware specially designated for diazomethane (e.g., with Clear-Seal joints) and should be used as soon as possible after preparation. Storage of diazomethane solutions (even at low temperature) is not advisable. All work with diazomethane should be conducted in a fume hood behind a safety shield, and appropriate impermeable gloves, protective clothing, and safety goggles should be worn at all times.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If this compound is inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, remove all ignition sources and close off the hood. Diazomethane solutions can be soaked up with a spill pillow or an absorbent material such as clay or vermiculite, placed in an appropriate container, and disposed of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
Small amounts of excess diazomethane can be destroyed by carefully adding acetic acid dropwise to a dilute solution of the diazomethane in an inert solvent such as ether at 0ºC. Excess diazomethane solutions and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
Preparative Methods
diazomethane is usually prepared by the decomposition of various derivatives of N-methyl-N-nitrosoamines. Numerous methods of preparation have been described,3 but the most common and most frequently employed are those which utilize N-Methyl-N-nitroso-p-toluenesulfonamide (Diazald®; 1),4 1-Methyl-3-nitro-1-nitrosoguanidine (MNNG, 2),5 or N-methyl-N-nitrosourea (3).
The various reagents each have their advantages and disadvantages, as discussed below. The original procedure6 for the synthesis of diazomethane involved the use of N-methyl-N-nitrosourea, and similar procedures are still in use today. An advantage of using this reagent is that solutions of diazomethane can be prepared without distillation,7 thus avoiding the most dangerous operation in other preparations of diazomethane. For small scale preparations (1 mmol or less) which do not contain any alcohol, a kit is available utilizing MNNG which produces distilled diazomethane in a closed environment.
Furthermore, MNNG is a stable compound and has a shelf life of many years. For larger scale preparations, kits are available for the synthesis of up to 300 mmol of diazomethane using Diazald as the precursor. The shelf life of Diazald (about 1-2 years), however, is shorter than that of MNNG. Furthermore, the common procedure using Diazald produces an ethereal solution of diazomethane which contains ethanol; however, it can be modified to produce an alcohol-free solution. Typical preparations of diazomethane involve the slow addition of base to a heterogeneous aqueous ether mixture containing the precursor. The precursor reacts with the base to liberate diazomethane which partitions into the ether layer and is concomitantly distilled with the ether to provide an ethereal solution of diazomethane. Due to the potentially explosive nature of diazomethane, the chemist is advised to carefully follow the exact procedure given for a particular preparation. Furthermore, since diazomethane has been reported to explode upon contact with ground glass, apparatus which do not contain ground glass should be used. All of the kits previously mentioned avoid the use of ground glass.
Handling, Storage, and Precautions: diazomethane as well as the precursors for its synthesis can present several safety hazards, and must be used with great care.8 The reagent itself is highly toxic and irritating. It is a sensitizer, and long term exposure can lead to symptoms similar to asthma. It can also detonate unexpectedly, especially when in contact with rough surfaces, or on crystallization. It is therefore essential that any glassware used in handling diazomethane be fire polished and not contain any scratches or ground glass joints. Furthermore, contact with certain metal ions can also cause explosions. Therefore metal salts such as calcium chloride, sodium sulfate, or magnesium sulfate must not be used to dry solutions of the reagent. The recommended drying agent is potassium hydroxide. Strong light is also known to initiate detonation. The reagent is usually generated immediately prior to use and is not stored for extended periods of time. Of course, the reagent must be prepared and used in a well-ventilated hood, preferably behind a blast shield. The precursors used to generate diazomethane are irritants and in some cases mutagens and suspected carcinogens, and care should be exercised in their handling as well.
- Related articles
- Related Qustion
Cyanogen bromide is a colorless to white crystalline solid with a penetrating odor. Cyanogen bromide is slightly soluble in water. Cyanogen bromide is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a f....
Sep 12,2019Chemical ReagentsSodium tripolyphosphate, also known as pentasodium triphosphate, pentasodium tripolyphosphate or sodium triphosphate, is used in a wide range of applications in the manufacture of cleaning products and food preservatives as well as in water....
Sep 12,2019Inorganic chemistryDiazomethane
334-88-3You may like
- How to synthesize Benzyl vinylcarbamate?
Mar 26, 2024
- Polypropylene and Polyvinyl chloride: Which one is better?
Mar 21, 2024
- How to synthesize Isoprene?
Mar 20, 2024
- DiazoMethane precursor
-

- $1.00 / 1KG
- 2020-01-14
- CAS: 334-88-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T