Diphenylmethane-synthesis and application
Dec 26,2019
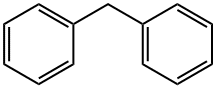
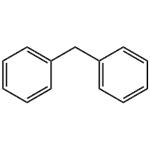
Diphenylmethane is an organic compound with the formula (C6H5)2CH2 (often abbreviated CH2Ph2). The compound consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. Diphenylmethane is a common skeleton in organic chemistry. The diphenylmethyl group is also known as benzhydryl, and it is prepared by the Friedel–Crafts alkylation of benzyl chloride with benzene (scheme 1) in the presence of a Lewis acid such as aluminium chloride [1].
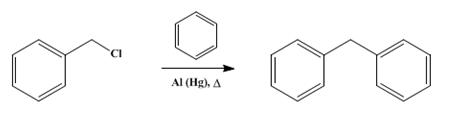
Scheme 1 Synthesis of Diphenylmethane
Diphenylmethane can be prepared from benzene and benzyl chloride with aluminum chloride, hydrogen fluoride, beryllium chloride, the double salt of aluminum and sodium chlorides, zinc dust, zinc chloride, or aluminum amalgam as a condensing agent. In another method, benzene and benzyl alcohol furnish diphenylmethane on treatment with boron fluoride, hydrogen fluoride, or beryllium chloride. Diphenylmethane has also been prepared from benzene, methylene chloride, and aluminum chloride, and from benzene, formaldehyde, ethanol, and concentrated sulfuric acid. The reduction of benzophenone to diphenylmethane has been affected by hydriodic acid and phosphorus, sodium and alcohol, and fusion with zinc chloride and sodium chloride. The condensation of benzylmagnesium chloride and benzene to diphenylmethane can be brought about by small amounts of magnesium and water [2].
The main application of diphenylmethane includes widely used in the synthesis of luminogens for aggregation-induced emission (AIE) and used in the preparation of a polymerization initiator, diphenylmethyl potassium (DPMK). It is one of the precursors in the synthesis of a dendrimeric polycyclic aromatic hydrocarbon (PAH), hexakis [4-(1,1,2-triphenyl-ethenyl) phenyl] benzene [3]. The following procedure is about the synthesis of DMPK.
The classical synthesis of DPMK is the indirect metallation via potassium naphthenide. n-Butyllithium (n-BuLi) solution (1.6 M in hexanes) and sec-butyllithium (sec-BuLi) solution (1.4 M in cyclohexane) were diluted and ampoulized on a high vacuum line. Lithium chloride (99.999%, LiCl,) was dried at 130 °C for 2 days and then diluted to the target concentration in THF and ampoulized under a reduced pressure of 10-6 mm Hg. Sodium (NaNaph) and potassium naphthalenide (K-Naph) were prepared by the reaction of the corresponding metal with naphthalene in THF at room temperature for 48 h. Diphenyl methyl potassium (DPMK) was prepared by the reaction of K-Naph with diphenylmethane in THF under high vacuum conditions at room temperature for 72 h. The concentration of DPMK was determined by titration using octyl alcohol and used for anionic polymerization. All initiators were sealed off under high vacuum into ampoules with break seals and stored at -30 °C.
Reference
[1] https://en.wikipedia.org/wiki/Diphenylmethane
[2] https://www.orgsyn.org/demo.aspx?prep=CV2P0232
[3] https://www.sigmaaldrich.com/catalog/product/aldrich/d209317?lang=en®ion=US
[4] https://pubs.acs.org/doi/10.1021/je050034s
[5] https://pubchem.ncbi.nlm.nih.gov/compound/7580
- Related articles
- Related Qustion
- Diphenylmethane: Natural Occurrence, Activity and Preparation Method May 8, 2024
Diphenylmethane, found in potatoes and soybeans, has therapeutic potential for insulin resistance via PPARα and PPARγ agonism, synthesized via Suzuki coupling.
- Diphenylmethane Serves as an Efflux Pump Inhibitor in Drug-Resistant Escherichia coli Jan 11, 2024
Diphenylmethane shows potential as an efflux pump inhibitor, enhancing antibiotic activity against drug-resistant bacteria, without affecting membrane permeability or post-antibiotic effects.
- Diphenylmethane: properties, applications and safety Sep 18, 2023
Diphenylmethane is a colorless liquid with aromatic odor, used in catalytic reactions and sonolysis, but requires caution due to potential irritant effects.
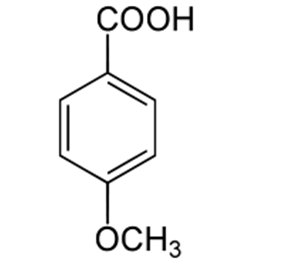
4-Methoxybenzoic acid, also known as p-anisic acid, 4-anisate or draconic acid, belongs to the class of organic compounds known as p-methoxybenzoic acids and derivatives. These are benzoic acids in which the hydrogen atom at position C-4 of....
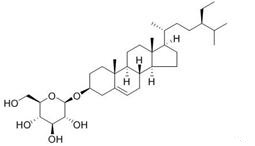
Dec 26,2019Chemical ReagentsDaucosterol, alias: Citoside, β-Sitosterol β-D-glucoside, and the molecular formula is C35H6. The compound of the formula (description column) belongs to a carotene compound.....
Dec 26,2019Plant extractsDiphenylmethane
101-81-5You may like
- Application research of 4-pyridinecarboxaldehyde
Nov 14, 2025
- Application research of 2,6-Dibromopyridine
Nov 12, 2025
- Application research of 2-Bromophenylboronic acid
Nov 12, 2025
- Diphenylmethane
-

- $10.00 / 1KG
- 2025-11-14
- CAS:101-81-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- Diphenylmethane
-
- $50.00 / 1KG
- 2025-09-25
- CAS:101-81-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Diphenylmethane
-

- $0.00 / 25KG
- 2025-09-23
- CAS:101-81-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month