Imatinib mesylate: Tyrosine kinase inhibitor
Oct 14,2019
Imatinib mesylate is a novel tyrosine kinase inhibitor and a derivative of phenylpyrimidine. About 95% of patients with chronic myeloid leukemia (CML) are Philadelphia chromosome-positive, that is, the proto-oncogene ABL of chromosome 9 is ectopic to the oncogene of chromosome 22 called the breakpoint clustering region (BCR). The two genes are recombined to produce the fusion protein p-210.
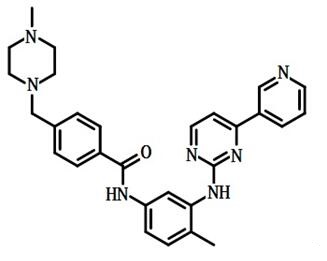
Figure 1: structural formula of Imatinib mesylate
Compared with the normal C-ABL protein p-150, p-210 has higher tyrosine kinase activity, which can stimulate leukocyte proliferation and lead to leukemia. Imatinib mesylate has a strong inhibition on the activity of ABL tyrosine kinase both in vitro and in vivo, specifically inhibit the expression of ABL and the proliferation of BCR-ABL cells, and thus can be used in the the treatment of CML. In addition, this drug also inhibits tyrosine kinases of platelet-derived growth factor (PDGF) and stem cell factor (SCF) receptors, and inhibits PDGF and SCF-mediated biochemical reactions, but does not affect the signal transduction of other stimulating factors such as epidermal growth factor.
Imatinib is available in 100- and 400-mg capsules for oraladministration and is indicated for the treatment of CML,gastrointestinal stromal tumors (GIST) that express Kit andacute lymphoplastic leukemia that is positive for thePhiladelphia chromosome. Bioavailability of the agent is nearly 100% by the oralroute. The agent is highly protein bound and metabolized tothe N-desmethyl derivative by CYP3A4-mediated removalof the piperazinyl methyl group. The resulting metabolite issimilar to the parent in activity. Elimination occurs primarilyin the feces, and the terminal half-life is 18 hours forthe parent and 40 hours of the N-desmethyl metabolite.Resistant forms of the TK are known, which have alteredamino acids that prevent binding. In addition, there may beincreased levels of the kinase itself. The drug is also a substratefor Pgp and an additional efflux transporter known asbreast cancer resistance protein (BCRP), both of which removethe drug from the cell. These transporters are also inhibitedby the agent as well. Severe side effects include ascites,neutropenia, thrombocytopenia, skin rash, andpulmonary edema. Less serious side effects include nausea/vomiting, heartburn, and headache but overall, the agentis better tolerated than most other medications used in treatingthe disease.
References
Sax, N. I., and Lewis, R. J. 1989. Dangerous properties of industrial materials, 7th ed.
New York: Van Nostrand Reinhold Company.
Arlien-Soborg, P., ed. 1992. Solvent neurotoxicity. Boca Raton, FL: CRC Press.
Sittig, M. 1985. Handbook of toxic and hazardous chemicals and carcinogens, 2nd ed.
Park Ridge, NJ: Noyes Publications.
Browning, E. 1965. Toxicity and metabolism of industrial solvents, 285–288. New
York: Elsevier Publishing Co.
Winder, C., and Stacey, N. H. 2004. Occupational toxicology. Boca Raton, FL: CRC
Press Inc.
- Related articles
- Related Qustion
Dazomet is a broad-spectrum pesticide that acts as fumigant nematicide. The decomposition of Dazomet in the soil releases methyl isothiocyanate, formaldehyde and hydrogen sulfide which kills the root nodule nematode and stem nematode.....
Oct 14,2019Chemical pesticides ?Gefitinib, an aniline quinazoline derivative, is an oral epidermal growth factor receptor’s (EGFR) tyrosine kinase inhibitor developed by AstraZeneca.....
Oct 14,2019InhibitorsImatinib mesylate
220127-57-1You may like
Imatinib mesylate manufacturers
- Imatinib Mesylate
-

- $650.00 / 1KG
- 2024-04-22
- CAS:220127-57-1
- Min. Order: 1KG
- Purity: High Density
- Supply Ability: 20 tons
- Imatinib Mesylate
-

- $0.00 / 1kg
- 2024-04-13
- CAS:220127-57-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000kg
- Imatinib Mesylate (STI571)
-

- $0.00 / 1g
- 2024-03-12
- CAS:220127-57-1
- Min. Order: 1g
- Purity: 98% HPLC
- Supply Ability: 1kg