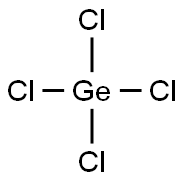Preparations and Reaction of Germanium tetrachloride
Oct 21,2019
Germanium tetrachloride is colorless liquid and decomposes when meeting with water. Soluble in ethanol and ether, easy to be soluble in dilute hydrochloric acid, insoluble in concentrated hydrochloric acid and concentrated sulfuric acid.It is used as a raw material for the preparation of semiconductor crucible. Alaoused as optical fiber dopant.
Preparation
Germanium(IV) chloride is prepared by reacting germanium metal with chlorine; or by treating germanium oxide, GeO2, with hydrochloric acid:
Ge + 2Cl2 → GeCl4
GeO2 + 4HCl → GeCl4 + 2H2O
Germanium(IV) chloride often is obtained as a byproduct of germanium metal production. The process involves heating germanium oxide, GeO2, with sodium chloride and coal. The vapors of germanium(IV) chloride and other volatile chlorides formed from the impurity metals are condensed. The product is isolated by fractional distillation. Further purification may be achieved by fractional distillation in 8N HCl and chlorine, or in the presence of other oxidizing agents in quartz stills.
Germanium(IV) chloride also is obtained by chlorination of germanium(II) chloride at ambient temperature. The reaction is rapid.
GeCl2 + Cl2 → GeCl4
Reactions
Germanium(IV) chloride reacts with water, hydrolyzing to germanium oxide and hydrochloric acid:
GeCl4 + 2H2O → GeO2 + 4HCl
The rate of hydrolysis is slower than the corresponding silicon analog, with hydrolysis occurring only partially. When heated with hydrogen at 1,000°C in a quartz reactor, it is converted into germanium(I) chloride, condensing onto the wall of the reactor:
2GeCl4 + 3H2→ 2GeCl + 6HCl
When vapors of GeCl4 are passed over germanium at elevated temperatures, the product is germanium(II) chloride, GeCl2:
GeCl4 + Ge→ 2GeCl2
Reaction with lithium aluminum hydride in ether forms monogermane, GeH4:
GeCl4 + LiAlH4 →GeH4 + LiCl + AlCl3
Reactions with antimony trifluoride, SbF3 in the presence of antimony pentachloride, SbCl5, form mixed halides of compositions: GeCl3F, GeCl3F2, GeCl2F2, and GeClF3.
Reactions with alcohols in the presence of an amine yield alkoxides:
GeCl4 + 4CH3OH + 4C2H5NH2 → Ge(OCH3)4 + 4C2H5N•HCl
Germanium forms six coordinate adducts, such as GeCl4(L)2 with many neutral ligands.
- Related articles
- Related Qustion
Dichloromethylsilane (methylsilicon dichloride; dichlorohydridomethylsilicon; methyldichlorosilane; methylhydrodichlorosilane; monomethyldichlorosilane) is an organic silicon substance with a molecular formula of CH3SiHCl2 or CH4Cl2Si.....
Oct 21,2019Organic Raw MaterialMethanethiol is mainly used to prepare methionine, lysine and tryptophan. Methanethiol is also used as raw materials in the manufacture of pesticide, herbicides and insecticides.....
Oct 21,2019Organic ChemistryGERMANIUM(IV) CHLORIDE
10038-98-9You may like
- Applications of Cuprous bromide
Nov 22, 2019
- Electronic and Optical Properties of Mercuric Iodate
Nov 4, 2019
- Uses of Lithium bromide
Nov 1, 2019
GERMANIUM(IV) CHLORIDE manufacturers
- Germanium chloride
-

- $15.00 / 1KG
- 2021-07-02
- CAS:10038-98-9
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton