Reactivity of Succinic Anhydride at Lithium and Graphite Electrodes
Nov 14,2019
Succinic anhydride (SA) is an useful electrolyte additive for high voltage cathodes but has also a negative impact on graphite (Gr) or Li anodes. For this reason, the Li/electrolyte and Gr/electrolyte interfaces were investigated at 20°C and 45°C using half or symmetrical cells. When SA is added at 1% by weight to the electrolyte (alkylcarbonate mixture + LiPF6), an increase in the Li/Li cell impedance at 20°C occurs owing to the formation of a resistive solid electrolyte interphase (SEI), composed of an inorganic inner layer and an organic outer layer, whereas at 45°C or in absence of SA, the interphase is more inorganic in nature. The reversible capacity of graphite, cycled in Gr/Li half-cells in the presence of SA, is very low at 20°C but almost ten times larger at 45°C. Cycling symmetrical Gr/Gr cells at 45°C indicates that Gr capacity is lower in the presence of SA in connection with the presence of an organic and polymer rich SEI. GC-MS analysis of the electrolyte after cycling shows that ethylene glycol bis-(alkylcarbonate) derivatives disappear when SA is present, owing to the ability of SA to react with lithium alkoxides to yield oligopolyester.
Whereas additives can be a suitable option for enhancing battery performances, their use for protecting cathodes is less common, due to the inherent instability of the surface film formed on the positive electrodes against dissolution, chemical or electrochemical transformation.4 Nevertheless anhydrides additives like succinic anhydride (SA) have a beneficial effect on Gr/LNMO cells,5 enhancing capacity retention, battery cycling and coulombic efficiency with a marked reduction of the self-discharge due to the creation of a thin protective layer on the positive electrode. As pointed out by V. Tarnopolskiy et al.,6 among many additives tested, only SA is efficient for using LMNO as a positive electrode and is able to reduce significantly the self-discharge of the electrode. In the same work, SA concentration in the electrolyte has been varied from 1% to 8% by weight, but only a slight influence was found on the performances of the Gr/LNMO cell. In order not to alter the main physicochemical characteristics of the electrolyte and prevent possible interferences with the anode, the lowest amount (1%) of additive maintaining the cell performances was selected for the present study. This feature reveals the importance of understanding the formation of passive layers to apprehend and optimize LIBs performances.
Materials
Graphite electrodes, purchased from Hohsen Corp., are composed of 79 wt % of Gr (active material), 14 wt % of carbon black (conductive material) and 7 wt % of polyvinylidene difluoride (PVDF), as binder, casted on Cu foil (10 μm thick) current collector. Graphite loading is 5.1 mg.cm−² and typically 50 microns thick.
Electrolyte preparation, 2032-type coin cells assembly and preparations for GC-MS analysis were all performed in argon-filled glove box ([H2O] < 0.5 ppm and [O2] < 20 ppm) at room temperature. Electrolytic solutions EMC-LiPF6 (1 M), EC/EMC (1:1 w/w)-LiPF6 (1 M) and EC/EMC (1:1 w/w)-LiPF6 (1 M) + 1% SA were prepared with EMC and EC purchased from Novolyte Technologies (purity ≥ 99.9%). Succinic anhydride (purity = 99.9%) and LiPF6 (Battery grade) were furnished by Across Organics and Fluorochem, respectively. Prior to their use, all solvents were dried with a moisture trap (ChemGenes Corp.) Lithium foil (0.75 mm thick) used as counter and/or reference electrode was purchased from Alfa Aesar.
Electrochemical tests
Li/Li cells were assembled with 2 lithium electrodes (∅ 16 mm) separated by a Viledon felt (Freudenberg, ∅ 16.5 mm) and a Celgard 2400 separator (∅ 16.5 mm) in 2032-type coin cells. Evolution of the interfacial resistance was evaluated by means of EIS operating in the range of 100 kHz to 2.5 mHz with an amplitude of 5 mV. EIS measurements were conducted at the beginning and every 25 hours for a total time of 3 days.
2032-type Li/Gr half cells were assembled using a Gr electrode (∅ 16 mm) and a lithium counter electrode (∅ 16 mm) separated by a Viledon felt (Freudenberg, ∅ 16.5 mm) close to Gr and a Celgard 2400 separator (∅ 16.5 mm) close to lithium. Three electrodes cells, purchased from Hohsen Corp. (HS-3E test cell), have a circular reference slot in which the lithium reference is inserted (16.15 mm < ∅ < 24 mm).
Symmetric Gr/Gr cells were built using two Gr electrodes lithiated up to the half theoretical capacity (0.5 mole of lithium per 6 mole of carbon) under a C/10 rate. As prepared Gr electrodes have a potential close to 0.1 V vs. Li/Li+. By this approach, the Gr/electrolyte interfaces are identical for both electrodes and the SEI is identical in composition and thickness. Half-lithiated Gr electrodes are removed from the cells, without washing. In order to measure the potential of each graphite electrode during the electrochemical tests, a three electrodes cell was built with (Li0.5C6)1 (as working electrode), (Li0.5C6)2 (as counter electrode) and metallic lithium as reference. Viledon (∅ 24 mm) and Celgard 2400 (∅ 24 mm) separators were placed between the working and reference electrodes. After building the cell with (Li0.5C6)1 and (Li0.5C6)2, the first full charge can be described as:
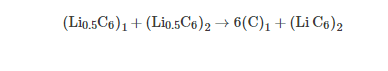
Results and Discussion
Electrode/electrolyte interface has been studied by electrochemical impedance spectroscopy (EIS) using symmetrical Li/Li cells. In order to follow the modifications brought to the interfaces by the reaction between Li and the EMC (LiPF6, 1 M) or EC/EMC (LiPF6, 1 M) electrolytes, measurements were carried out just after the cell assembly and then every 25 hours. The imaginary and real part of the complex impedance were measured at 20°C and plotted as a Nyquist diagram in Fig. 1 for the EMC (LiPF6, 1 M) and EC/EMC (LiPF6, 1 M) electrolytes containing or not 1% of SA.
The impedance diagram exhibits a well-defined slightly depressed half-circle in the high-medium frequency range which reflects the interfacial impedance including SEI film and charge transfer resistances. In the low frequency range (below 1 Hz), an ill-defined curve is observed which cannot be clearly attributed to a diffusion process. In EMC electrolyte, little variations occur at the interface as the circle diameter and the frequency at the apex of the half-circle, related to the apparent time constant of the system (τ = RC) remains almost constant with time. This means that the SEI is formed rapidly and is stable. In the EC/EMC electrolyte, the lithium interphase is not as stable as the half-circle increases in diameter with time meaning that the SEI layer is growing upon contact between electrolyte and lithium. Nevertheless the frequency at the top of the half-circle in the EC/EMC electrolyte is not affected by the electrode/electrolyte exposure time. As a partial conclusion, the EC component of the blended electrolyte is more reactive toward lithium than EMC.
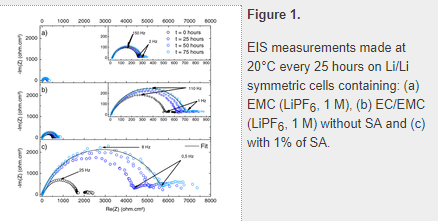
When SA is added to the EC/EMC electrolyte, the half-circle becomes almost ten times larger in diameter and expands very fast during the first 24 h of electrode exposure to the electrolyte. Moreover, the frequency at the apex of the half-circle shifts from 25 Hz at t = 0 to 8 Hz at t = 75h as pointed out on the graph reported in Fig. 1. This shift in frequency is linked to a variation in the time constant of the equivalent R//C circuit and indicates a corresponding increase in RC values. This point will be discussed in the following paragraph.
Effect of the temperature
The impact of raising the temperature from 20°C to 45°C on the apparent interfacial resistance Ri is shown in Fig. 4 for both studied electrolytes. A linear dependence of Ri with the time square root indicates that the SEI growth is controlled by diffusion of electrolyte components. A change in the SEI structure or depletion of SA after 25 h of contact with the electrolyte accounts for the slope break, suggesting that the new structure has a slower growth or a lower conductivity.
A beneficial effect of rising the temperature to 45°C is the decrease of the interfacial resistance Ri≈RSEI. After 100 hours of storage, Ri value decreases from 6000 Ω cm2 at 20°C to 1000 Ω cm2 at 45°C, a value which is similar to that obtained without SA. This decrease cannot only be interpreted on the basis of an increased ionic conductivity of the mineral layer (according to the CSL model) but rather on a structure change of the outer layer of the interphase. It is very likely that the outer layer which is mainly polymeric undergoes a change in porosity or, in amorphous regions, a glass transition phenomenon. This hypothesis will be supported thereafter by experimental results.
Conclusions
The effect of succinic anhydride, dissolved in an EC/EMC-LiPF6 electrolyte, on lithium and graphite electrode interfaces has been studied with the aim to determine the mechanisms implied. It has been first shown that the addition of 1% of SA to the electrolyte increases the impedance of the lithium/electrolyte interface drastically at 20°C. The SEI growth at the lithium/electrolyte interface is controlled by diffusion with a strong dependency on the temperature as at 45°C the SEI growth rate is slower. In the presence of SA in the electrolyte, the Gr reversible capacity is as low as 25 mA h g−1 at 20°C but ten times larger when the cell is operated at 45°C. This is explained on the basis of a large ohmic drop associated with a resistive SEI, which decreases when the temperature is set to 45°C. In order to avoid any influence of the film formed at the lithium counter electrode in half cells, which is very resistive in the presence of SA, the Gr/electrolyte interface was studied by means of symmetrical Gr/Gr cells at 45°C. Cycling experiments confirm that at the first cycle the full Gr capacity is recovered when SA is not added to the electrolyte. In such symmetric cell, the reversible capacity fades upon cycling for an unknown reason but in any case, capacity values remain lower in presence of SA. This proves that SA has globally a negative impact on the Gr/electrolyte interface as well as the Li/electrolyte. XPS measurements show that the composition of the SEI formed on Gr is richer in organic and polymeric compounds and poorer in LinFm clusters or lithiated species when SA is added, their scarce presence hindering lithium conduction through the SEI film. GC-MS analysis show that, after cycling in presence of SA, the electrolyte does not contain any ethylene glycol bis-(alkylcarbonate) derivatives which are commonly observed in standard alkylcarbonate based electrolytes and attributed to solvents reduction. A mechanism enlightening the role of SA during SEI formation is proposed.
- Related articles
- Related Qustion
- Succinic anhydride: applications in diverse industries Jul 3, 2023
Succinic anhydride is a compound that is commonly used in various applications.
The formation of acetoin (acetylmethylcarbinol) by various tissues has been shown to be a synthetic process, the 4-carbon ketol originating from pyruvic acid in bacteria (1, 2), from pyruvic acid and acetaldehyde in yeast (3) and animal tis....
Nov 14,2019Organic ChemistrySodium thiosulfate (STS) is an industrial chemical which also has a long medical history. It was originally used as an intravenous medication for metal poisoning. It has since been approved for the treatment of certain rare medical conditio....
Nov 14,2019Inorganic chemistrySuccinic anhydride
108-30-5You may like
- How to synthesize Benzyl vinylcarbamate?
Mar 26, 2024
- Polypropylene and Polyvinyl chloride: Which one is better?
Mar 21, 2024
- How to synthesize Isoprene?
Mar 20, 2024
Succinic anhydride manufacturers
- Succinic anhydride
-

- $1.00 / 25kg
- 2024-04-25
- CAS:108-30-5
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 2000mt/year
- Succinic anhydride
-

- $0.00 / 1kg
- 2024-03-27
- CAS:108-30-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000kg
- Succinic anhydride
-
- $6.00 / 1KG
- 2024-01-18
- CAS:108-30-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available