Use of Butyric acid
Dec 3,2021
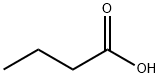
Butyric acid is a four-carbon acid with an unpleasant and obnoxious odor, with a butter-fat taste. It is a clear and colorless oily liquid and occurs in butter and animal fat as the glycerol ester. n-Butyric acid is manufactured in enclosed, continuous equipment by the catalyzed air oxidation of butyraldehyde. It can be produced via fermentation of organic materials by anaerobic bacteria in the large intestine, and remains a major energy supply for colonic cells. Butyric acid is involved in a range of processes in the body such as native immunity, induction of apoptosis, and regulation of water and electrolytes. It is an important metabolite in the breakdown of carbohydrates, fats, and proteins and is mainly used as an industrial intermediate and food additive.

Use
Butyric acid is used in the manufacture of cellulose acetate butyrate (CAB) plastics. CAB sheets are used for thermoformed signs, blister packaging, and in goggles and face shields. Molded CAB is used to make pen barrels, eyeglass frames, and screwdriver handles. CAB is a component in acrylic enamel used in automotive manufacturing coatings. Some butyric acid is used to make butyroperoxides and herbicides.
It is also used as an intermediate for pharmaceuticals, emulsifiers, and disinfectants, as a leather tanning agent, and a sweetening agent in gasoline. It is used in the synthesis of butyrate ester perfumes and in the manufacture of esters, some of which serve as the bases of artificial flavoring ingredients in certain liquors, sodawater syrups, and candies. It is also used as a food additive in butter, cheese, butterscotch, caramel, and fruit and nut flavors. Butyric acid is classified as a generally considered as safe (GRAS) material by the US Food and Drug Administration (US FDA). Butyric acid is also used in the preservation of high moisture wheat grains to prevent fungal deterioration. In therapeutics, butyric acid is used as a histamine antagonist. Due to its powerful odor, it has also been used as an additive in fishing bait. The antiangiogenic and antineoplastic activities of the butyric acid prodrugs, AN-7 and AN-9, were demonstrated in vitro by inhibition of proliferation and vascular tube formation, enhanced apoptosis, and inhibition of 22Rv-1 cell migration. The inhibition of tumor growth and metastasis and the low toxicity of AN-7 and AN-9 have been demonstrated in preclinical studies and clinical trials.
Generally, prodrugs of butyric acid rapidly penetrate into cancer cells where they hydrolyze to butyric acid and aldehyde. The most active prodrugs in this family of acyloxyalkyl esters are the ones that release formaldehyde and they possess anticancer properties superior to those elicited by the homologous prodrugs that release acetaldehyde. The potential use of butyric acid prodrugs for the treatment of neoplastic diseases and b-globin disorders has been suggested.
Environmental Fate
Butyric acid is not environmentally persistent as it is biodegradable in aqueous media, volatilizes from surface waters at a moderate rate, and readily undergoes photodegradation in the atmosphere. n-Butanoic acid may be susceptible to biodegradation in terrestrial and aquatic environments based on the observed degradation of 72% after 5 h when incubated with activated sludge.
At an initial concentration of 100 mg l-1, n-butanoic acid displayed a 72% theoretical biological oxygen demand (BODT) after 5 h when incubated with activated sludge. n-Butanoic acid at an initial concentration of 5 ppm displayed a BODT of 76.6% in freshwater and 72.4% in seawater after 5 days. n-Butanoic acid had a BODT of 17.4, 23.8, 26.2, and 27.7% after 6, 12, 18, and 24 h, respectively, when incubated with an activated sludge seed at an initial concentration of 500 ppm. In a screening study, the BODT of n-butanoic acid was 46, 48, and 58% after 2, 10, and 30 days, respectively, using a sewage seed. In a screening study using a sewage seed, n-butanoic acid had a 5-day BODT of 72–78% and a 20-day BODT of 92–99%.
Butyric acid must be separated from strong oxidants, strong bases, food, and feedstuffs for long-range transport (UN Hazard Class 8; UN Packing Group III; stable during transport). It should be stored in a cool, dry, well-ventilated location, away from any area where fire hazard may be acute. Outside or detached storage is preferred, separate from oxidizing materials, heat, oxidizers, and sunlight.
Mechanism of Toxicity
The most probable mechanism of toxicity is the formation of an acid proteinate following exposure to high concentrations. Such complexes result in an inhibition of protein function and disruption of cellular homeostasis. Butyric acid induces apoptosis by production of ceramide and reactive oxygen species in the mitochondria followed by activation of JNK in mitogen activated protein (MAP) kinase cascades. Butyric acid has two contrasting functional roles. As a product of fermentation within the human colon, it serves as the most important energy source for normal colorectal epithelium. It also promotes differentiation of cultured malignant cells.
A switch from aerobic to anaerobic metabolism accompanies neoplastic transformation in the colorectum. The separate functional roles for n-butyrate may reflect the different metabolic activities of normal and neoplastic tissues. Deficiency of n-butyrate, coupled with the increased energy requirements of neoplastic tissue, may promote the switch to anaerobic metabolism. n-Butyrate was previously found to increase epidermal growth factor receptor binding in primary cultures of rat hepatocytes.
It was shown that butyrate and dexamethasone synergistically modulate the surface expression of epidermal growth factor receptors. The butyrate-induced enhancement of highaffinity epidermal growth factor bindingwas slight in the absence of glucocorticoid, but was strongly and dose-dependently amplified by dexamethasone. Butyrate counteracted the inhibition by insulin of the dexamethasone-induced increase in epidermal growth factor binding. The results indicate that the glucocorticoid has a permissive effect on a butyrate-sensitive process that determines the surface expression of the high-affinity class of epidermal growth factor receptors.
- Related articles
- Related Qustion
- Butyric acid: Production, applications and pharmacodynamics Jul 7, 2023
Butyric acid is a straight-chain alkyl carboxylic acid and its isomer is isobutyric acid (2-methylpropanoic acid). Salts and esters of butyric acid are known as butyrates or butanoates.
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colourless flammable liquid with an unpleasant smell. It is miscible with most organic s....
Dec 2,2021Organic Chemistry3-Quinuclidinyl benzilate (QNB) — IUPAC name 1-azabicyclo[2.2.2]octan-3-yl hydroxy(diphenyl)acetate; US Army code EA-2277; NATO code BZ; Soviet code Substance 78— is an odorless and bitter-tasting military incapacitating agent.BZ is an anta....
Dec 3,2021APIButyric Acid
107-92-6You may like
- Butyric Acid
-

- $10.00 / 1kg
- 2025-04-23
- CAS:107-92-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 mt
- Butyric Acid
-

- $278.00 / 200KG
- 2025-04-02
- CAS:107-92-6
- Min. Order: 5000KG
- Purity: 99.5% Min
- Supply Ability: 1000 tons/month
- butyric acid
-

- $0.00 / 25KG
- 2025-03-21
- CAS:107-92-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month