Uses of Beta-propiolactone
Nov 24,2021
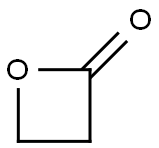
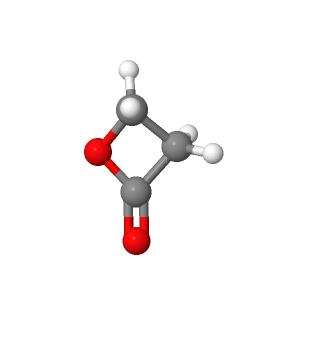
Beta-propiolactone is a colorless liquid with a strong, slightly sweet odor. It may occur naturally, but no clear documentation of its occurrence in nature was found, and it must be synthesized for commercial purposes. Beta-propiolactone is unstable at room temperature but stable when stored at 5 °C in glass containers.
Uses
Beta-propiolactone‘s tendency to be unstable and react with other molecules in the vicinity is responsible for both its toxicity and its usefulness. Significant commercial production of beta-propiolactone took place during the late 1950s through the mid-1970s, when it was widely used in chemical synthesis in reactions with other molecules to produce new chemicals. All lactones are characterized by a ring structure consisting of two or more carbon atoms – as can be seen from its structure, beta-propiolactone has three in its ring – and a single oxygen, coupled with an adjacent ketone. The fewer the carbons in the ring, the more ‘strained’ is the ring structure and the more unstable and reactive its characteristics. When the ring bonds break, the betapropiolactone molecules attach to other nearby molecules.
Once a commercially important industrial chemical in the United States, more than 85% of the beta-propiolactone produced was used to manufacture acrylic acid and esters. Preparation of commercial acrylates involved reacting betapropiolactone with ethylene cyanohydrin. The instability of beta-propiolactone, coupled with the discovery of other more desirable chemical synthesis methods, led to its replacement by other starting materials in newer manufacturing methods.
A variety of sources report that beta-propiolactone has also been used for disinfecting blood plasma, human and veterinary vaccines, tissue grafts, surgical instruments, enzymes, nutrient broth and milk; as a vapor-phase disinfectant in enclosed spaces; and as a sporicide against vegetative bacteria, pathogenic fungi, and viruses. It has also been used by shipboard military personnel as a disinfectant/decontaminant. It is sometimes also used in laboratory research. Because it is no longer used as a sterilant in medical procedures or in food, the potential for exposure of the general population is increasingly limited.
Environmental Fate
It is soluble in water (370 g l-1 at 25°C) and miscible in other common organic solvents including acetone, chloroform, diethyl ether, and ethanol (Log Kow 0.462). Hydrolysis occurs in water where the half-life in aqueous media at 25°C is approximately 3.5 h. If released to soil, relatively rapid hydrolysis can be expected to occur in the presence of moisture. Significant evaporation may occur from dry surfaces. With a vapor pressure of 3.4 mm Hg at 25°C, it can also vaporize into the air as temperature rises. If released to the atmosphere, beta-propiolactone is expected to exist in the gas phase, where it may be relatively more persistent in the absence of moisture than it is in aqueous media. The half-life for the reaction with photochemically produced hydroxyl radicals was estimated to be a relatively slow rate of 45 days in the atmosphere.
Mechanism of Toxicity
Beta-propiolactone is sometimes classified as a direct-acting alkylating agent capable of attaching to DNA and forming DNAadducts. That property probably accounts for its mutagenic activity in a wide variety of in vitro and in vivo test systems using both somatic and germ cells. It can also react with amino acids of enzymes and other proteins, including blood proteins like albumin. If given by itself intravenously, it causes liver necrosis and kidney tubular damage, but if it is allowed time to react with proteins before injection, then toxicity is much reduced.
- Related articles
- Related Qustion
Beryllium (Be), formerly (until 1957) glucinium, chemical element, the lightest member of the alkaline-earth metals of Group 2 (IIa) of the periodic table, used in metallurgy as a hardening agent and in many outer space and nuclear applicat....
Nov 24,2021Inorganic chemistryFrench physician Jean Sterne published the first clinical trial in 1957 suggesting metformin as a treatment for diabetes. The United Kingdom introduced this drug in 1958 followed by Canada in 1972, and the United States in 1995. As of 2010,....
Nov 24,2021Chemical Reagents2-Oxetanone
57-57-8You may like
- Benzhydrol:Melting point,Uses,Hazards
Mar 22, 2024
- What is Methoxypolyethylene glycol amine used for?
Mar 14, 2024
- What is 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine?
Mar 14, 2024
- 2-Oxetanone
-
- $8.00 / 1KG
- 2024-04-09
- CAS:57-57-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 2-Oxetanone
-

- $0.00 / 1kg
- 2023-12-04
- CAS:57-57-8
- Min. Order: 1kg
- Purity: 99.5%
- Supply Ability: 5000kg
- 2-Oxetanone
-

- $0.00 / 25KG
- 2023-06-27
- CAS:57-57-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month