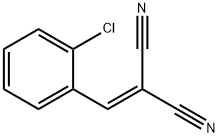
Uses of CS
Jan 12,2022
CS, or o-chlorobenzylidene malononitrile, is the current major riot control agent (RCA) used by U.S. military forces. It was originally synthesized in 1928 by Corson and Stoughton, and the U.S. Army designated the compound ‘CS’ for the authors’ initials. CS replaced CN, chloroacetophenone, in 1959 as the U.S. Army’s premier RCA due to its higher safety ratio over CN. CS is more effective as an RCA, that is to say, more potent and safer than its predecessor CN. CS can be disseminated pyrotechnically, or in the cases of CS1 and CS2, in powder formulations.

Uses
CS is used as a nonlethal or less-than-lethal chemical in riot control situations, to distract, deter, incapacitate, disorient, or disable disorderly people, to clear facilities, areas, deny areas, or for hostage rescue. It can also be used in peacekeeping operations. It is also used in military training as a confidence builder for the protective mask. In addition to the nonpersistent form of CS, two hydrophobic variations were created, CS1 and CS2. CS1 is a micronized powder formulation containing 5% hydrophobic silica aerogel, which can persist for up to 2 weeks in normal weather conditions, and CS2 is a siliconized microencapsulated form of CS1 with a long shelf life, persistence, resistant to degradation, and ability to float on water, which could restrict or deny the use of water for military operations. CS is commonly used as an RCA and a simulant for training. Members of military organizations and law enforcement agencies are routinely exposed to heated CS during training. The heat vaporizes the CS for dispersion, which then condenses to form an aerosol.
Environmental Fate
Tests conducted at Eglin Air Force Base near Ft. Walton Beach, Florida, concluded that CS in soil has a ‘conservative’ half-life of 3.9 days. Degradation of CS increases with soil and air moisture, and with light exposure as it undergoes a slow hydrolysis process. Olajos (2004) concluded that CS degrades to o-chlorobenzaldehyde and malononitrile, the former of which is ultimately converted to catechol under aerobic conditions. Nitriles such as the malononitrile degradation product of CS have a short half-life in soil and can be converted readily to organic acids. Anaerobically, microalgae have been reported to transform CS breakdown products into benzoate. The U.S. Army Edgewood Research Development and Engineering Center (USAERDEC) reports a solubility of 200 mg l-1.
CS concentrations attenuate in the atmosphere through three processes: reaction with hydroxyl radicals, hydrolysis reactions with atmospheric humidity, and deposition of particulate CS. It should be noted that vapor pressure increases with temperature, as should be expected. At 20°C, CS vapor pressure is 3.5×10-5 mmHg, and at 60°C, it is 5–7 mmHg.
Mechanism
CS as well as CN is an SN2-alkylating agent with activated halogen groups that react readily at nucleophilic sites. The prime targets include sulfhydryl-containing enzymes such as lactic dehydrogenase. In particular, CS reacts rapidly with the disulfhydryl form of lipoic acid, a coenzyme in the pyruvate decarboxylase system. It has been suggested that tissue injury may be related to inactivation of certain of these enzyme systems. CS causes the release of bradykinin, which can cause pain without tissue injury. The initial response to the inhalation of CS or other sensory irritants is consistent with the Kratschmer reflex and the Sherrington pseudoaffective response. These aerosols stimulate the pulmonary irritant receptors to produce bronchoconstriction and increased pulmonary blood volume by augmenting sympathetic tone. The chlorine atoms released from CS on contact with skin and mucus membranes are reduced to hydrochloride acid, which can cause local irritation and burns.
- Related articles
- Related Qustion
- [(2-Chlorophenyl)methylene]malononitrile: Burn Injury of Exposure and its Mechanisms Apr 18, 2024
Exposure to [(2-Chlorophenyl)methylene]malononitrile can cause burn injuries through flame, contact, and chemical mechanisms, necessitating diverse medical treatments.
- [(2-Chlorophenyl)methylene]malononitrile: properties, applications and safety Oct 18, 2023
[(2-Chlorophenyl)methylene]malononitrile is widely used for riot control, causing severe irritation but with low lethality.
Cromolyn is a synthetic analog of the plant extract khellin that was first developed in the late 1960s and introduced into clinical practice the following decade. It is continued to be used widely in the treatment of allergic rhinitis, asth....
Jan 12,2022Biochemical EngineeringCumene is a common name for isopropylbenzene, an organic compound. Cumene is a volatile colorless liquid at room temperature with a characteristic sharp, penetrating, aromatic odor. It is insoluble in water but is soluble in alcohol and man....
Jan 12,2022Organic Chemistryo-Chlorobenzylidene malononitrile
2698-41-1You may like
- Benzhydrol:Melting point,Uses,Hazards
Mar 22, 2024
- What is Methoxypolyethylene glycol amine used for?
Mar 14, 2024
- What is 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine?
Mar 14, 2024
o-Chlorobenzylidene malononitrile manufacturers
- 2-Chlorobenzylidenemalononitrile
-

- $1.00 / 1kg
- 2023-09-07
- CAS:2698-41-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
- 2-Chlorobenzylidenemalononitrile
-

- $1.00 / 1BOU/Drum
- 2020-11-27
- CAS:2698-41-1
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1kg
- CS gas
-
- $1.00 / 1KG
- 2020-02-01
- CAS:2698-41-1
- Min. Order: 1KG
- Purity: Min98% HPLC
- Supply Ability: G/KG/TON